Posting to Google Drive and adding blog comments is now required for an invitation to the CCI Solar Annual Meeting in January. We’ve got a few more teams regularly updating, but hopefully we’ll have everyone regularly updating soon.
Beckman HS’s 4 groups are working with a UV ozone cleaner and testing various concentrations and combinations of iron, copper and nickel nitrates. Group 1 did the UV treatment once more on 0.04 M iron (III) nitrate because the last test had inaccurate spotting. Also, for the UV treatment, Group 1 used a new mask that only applied UV treatment on the spots, and checked to see the difference. Additionally, Group 1 made two new plates with 0.04 M copper nitrate and 0.04 M nickel nitrate to check for better results when applied with UV treatment during the next meeting. Group 2 tested and compared two plates of 0.1 M iron (III) nitrate with UV treatment and without UV treatment to see how well the UV treatment worked. Analysis is yet to be determined. Group 3 made a plate with spots with different ratios of 0.1 M iron (III) nitrate, 0.1 M copper (II) nitrate, and 0.1 M nickel (II) nitrate because of the possibility of better results when mixing multiple metal oxides, and the ratios were 1:1:1, 1:1:2, 1:2:1, and 2:1:1. During the next meeting, this plate will be tested and run with UV treatment, and further analysis will be shared. Group 4 did UV treatment on the 0.05 M iron (III) nitrate plate that was spotted and heated at the last meeting to test for results. Also, they spotted a 0.03 M Iron (III) nitrate plate but could not do UV treatment on the plate to see which molarity had better results due to a shortage of time.
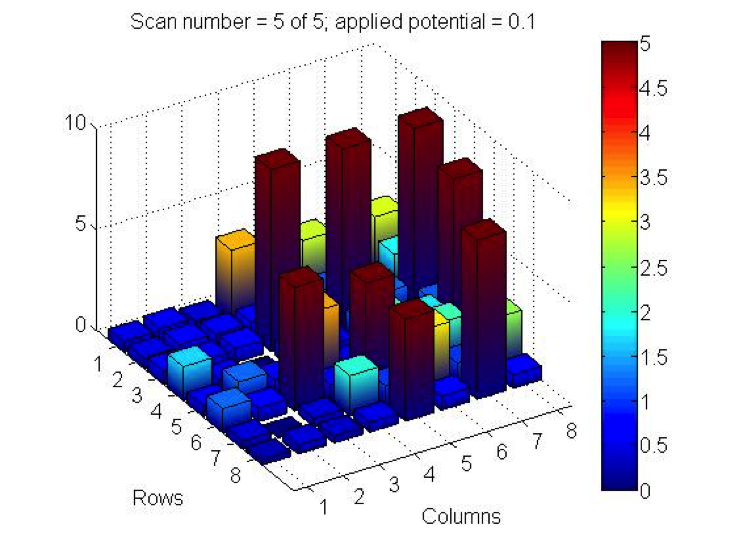
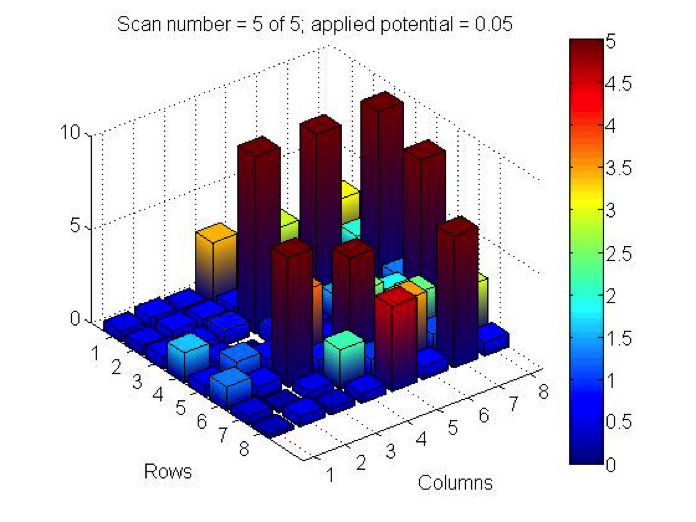
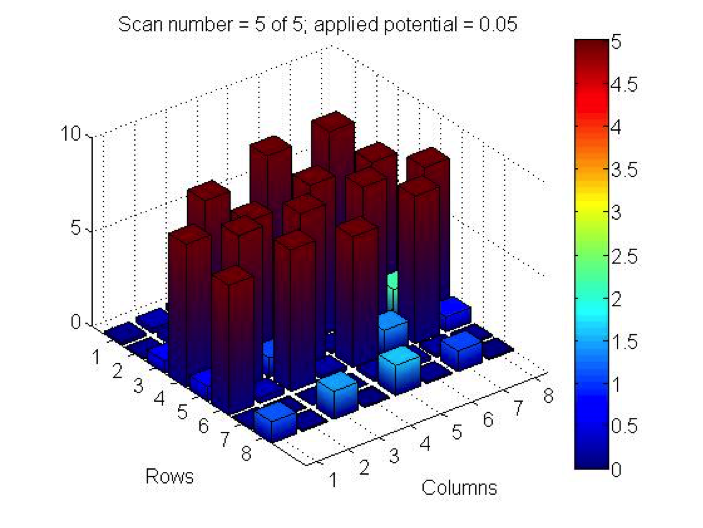
PCC did some testing this week of 2 plates they made last month and had some incredible results! Benjamin tested iron nitrate, lead nitrate and bismuth nitrate in varying combinations. Page tested combinations of iron nitrate and bismuth nitrate, though she wasn’t at the meeting to confirm exact ratios. While the dark current of both plates wouldn’t settle to the 0.5 threshold (1.2, 0.9 for Benjamin’s plate and 1.8 for Page’s), the results show current far surpassing that background. Check out their results below:
The spots containing bismuth nitrate gave the highest measurements of photoactivity. The pure bismuth(II) nitrate spots measured close to 9, while iron(III) nitrate spots were around 3 and 4. The lead(II) nitrate spots gave very low values of approximately 1-2. Combining bismuth(III) nitrate with other solutions lowered the photoactivity of the bismuth nitrate.
Poly is still struggling with getting the HARPOON kit to be purged correctly and stay oxygen free for testing. Franklin HS- please send them some advice in the comments since you have the most experience with the kit! All they see if green despite having a purged solution from their mentor and a blanket of argon gas on top of the solution. Have you had any success with the mesh in the lab recently? Do we need new ones?
San Marino’s Red Team prepared the 2 plates (one electroplated and one drop casted) for testing with the SEAL kit. They applied 0.13 Volts and got about 0.015 microamps of dark current. Both plates only registered results in the blue region. However, much of the deposited metal oxides on the plates flaked off during testing. They speculate the reason for this is that they used a 1 molar concentration of some of the hydrated metal compounds instead of the normally used 0.1 molar concentration. Therefore, they plan on using 0.1 molar from now on when they do electroplating. For future meetings, they might also start focusing on the juice from juice research, where we will examine varying types of juices and their efficiency in creating a current flow.
Team KEN from Mayfield had poor luck last time repeating successful results from last year with FeZn. They theorize that the Fe solution last year was contaminated with nickel, and so might have skewed the results as FeNi have been known to work well. They created a new FeZn plate and FeNiZn and FeNi plates to determine them if the addition of zinc helped the results, and also if the solution was contaminated. Team RAM tested a CuW plate with moderate results (current in the light blue region). Unfortunately the second plate they tested with CuW, CoW, and ZnW had technical issues and there were no results. Try re-epoxying and test again girls! Lastly, team SEA tested a couple plates of their colored and non-colored metal salt combinations. While they didn’t have high current output, the 1:2 ratio of colored to uncolored metals produced slightly higher results than the plate with the 2:1 ratio of colored to uncolored. Team SEA has decided to work more with the more successful ratio, and also have decided to make new plates with a 1:3 or 1:4 ratio of colored to uncolored metals in the future.
Congratulations on all the hard work everyone! It’s exciting to start seeing results and the progress everyone is making!

@PCC: We too are conducting research on Bi(NO3)3! We have noticed that repeated tests of the same bismuth spots lead to photo activity degradation. Have you noticed the same?
A significant amount of our plates have contained iron nitrate, but we haven’t received exceedingly high results. Are there any special techniques you used in order to make such successful plates? Thank you!