SoCal Infantry
Welcome to the Southern California SEAL infantry! SoCal SEAL students check here for important information regarding the SEAL program at Caltech
Important dates for 2019-2020 school year:
SEAL Kickoff: October 5, 2019 10am-12pm
SEAL Con XI: May 9, 2020 10am-2pm
2019-20 SEAL Roster
Every member of a SoCal SEAL team needs to sign-up on the roster. This ensures you will receive communications about upcoming events and helps us keep track of our membership. This also is how we make certificates for SEAL Con!
SEAL Permission Slip
All SoCal SEAL students need to fill out this permission slip and return it back to Kitty Cahalan either via email (kcahalan@caltech.edu) or in person through their mentor. Students under 18 must have the form signed by a parent or guardian.



Archives of SoCal SEAL Blog
Click on a blog post to view and add comments
Week of 4/10-4/16
Beckman Group 1 tested the plate with 1:1, 2:1, 3:1, 4:1 ratios of iron to aluminum since they increased the amount of aluminum last time so they can compare the effects of increasing ratio of iron to aluminum to effects of increasing aluminum to iron. Group 2 tested their plate (BHS-2-71-B) which consisted of the Bismuth Nitrate solution and Iron standard solution. The ratios they used are 1:12, 1:15, 4:3, and 5:3. The results they gained were good as the range was from 3-5. They plan to make a replica of plate BHS-2-71-B to verify results. They did not test plate BHS-2-71-A which consisted of the standard Iron Nitrate and Bismuth solution with ratios of 1:1, 1:19, 1:18, and 2:1 because the spots were too small to be tested on after the plate was baked. Group 3 tried using HCl acid to dissolve the 0.04 M Bismuth solution but it failed to dissolve completely. They are using a hot plate in order to try dissolving the 0.04 M Bismuth solution. Lastly, Group 4 tested the plate that was UV treated for 10 minutes. It is part of their trials to determine if UV treatment longer than five minutes has benefits regarding the coffee ring effect and testing plates.
San Marino Blank Team made a new plate with varying ratios of Fe(NO3)2 and Zn(SO4). All of the Caltech SEAL kits are currently out for repairs, so no testing was possible this week. Hopefully they will be all fixed soon!
Weeks of 3/27-4/9
We’ve had a few weeks of slow activity due to Spring Break, but we’re back with some more updates. Only one month left until SEAL Con!
Beckman Group 1 is testing their plate BHS-1-52, which they epoxied last week. The plate consists of ratios of 1:1, 2:1, 3:1, and 4:1 of the iron and aluminum nitrate solutions. Group 2 epoxied their plate (BHS-2-71-B), which consists of the bismuth nitrate solution and iron standard solution. The ratios they used are 1:12, 1:15, 4:3, and 5:3. They will be making the plate BHS-2-71-A, which also consists of the standard iron nitrate and bismuth solution. The ratios they are using are 1:1, 1:19, 1:18, and 2:1. Group 3 started to make a plate with iron standard and bismuth nitrate. The ratios they will be using are 1:2, 2:1, and 1:1.
Poly Group 1 epoxied the two plasma cleaned 0.1M Fe(NO3)3 plates and two plasma-cleaned cobalt acetate plates. They also spotted one 0.1M MnSO4 plate with 0.1M Fe(NO3)3 to see if layering the plates with Fe(NO3)3 before placing them in the kiln would make the spots less likely to spread. Finally, they layered 0.1 Fe(NO3)3 on one fired cobalt acetate plate and one fired plate that had both MnSO4 and cobalt acetate. There was less spreading on the fired plates, maybe because last time pushing the pipette all the way put too much Fe(NO3)3 solution on the spots? This time, they only pushed half way, and it worked out better.
Group 2 scanned Fe plates that were not treated with plasma. The results were pretty solid, but compared to the Fe plates treated with plasma these results were not as strong. Next time, they are planning to test the Co and Mn plates layered with Fe(NO3). They hope to have more data to show the benefits of plasma cleaning.
San Marino Blank Team tested the plate made last week. The overall goal was to make sure that trends from the first two trials are actually due to the material and not due to residue current/drift. Putting the same concentrations in rows and getting trends means it is actually due to material, not drift. Putting same concentrations in columns and getting trends in rows further verifies it is not due to drift. When you check the dark potential after applying a current, the SEAL kit checks whole plate’s current (without light activation). When it checks spots with the LED light, it checks the whole current, combining the dark current and the light-induced current. If the dark current fluctuates between spots, you would not be able to tell if variability between spots is due to different photo-activities and changing dark currents. By changing the orientation of the spots to differ from the column testing of the SEAL Kit, you can reduce the variable of time between testing spots.
Green Team made a new copper and nickel plate and fired it at 450°F.
Week of 3/20-3/26
Canyon Crest was able to test 5 plates and got good results from several of them (see below). They made two new plates using the new spotting template that was made last week, but will have to wait until they are tested to see how much of a difference it makes. One of the plates hopefully will build off recent successes with iron and potassium, while the other is an interesting experimental plate consisting of sodium, manganese, and iron inspired by new battery technology (see 2/27 entry).
KMnO4, LiCl, Fe(NO3)3 (80:0:10, 90:0:0, 60:30:0) data was so promising that they created a plate with other potassium and iron compounds: FeCl2, K4Fe(CN)6, K2Cr2O7. Also made a plate with NaNO3, Mn(NO3)2, FeCl3 to test any effect of Manganese in the potassium manganate.
(NH4)3VO3, Fe(NO3)3, K2Cr2O7 (0:70:20) reinforces the previous data that suggested a positive combination of ~3:1 Iron:Potassium. However, the ammonium vanadate clearly had little impact on the data. Because of this and other null results from using complex ions (namely ammonium cerium nitrate), they are going to avoid using obscure complex ions in order to test the central atom.
San Marino Blank Team made a new plate with 0.1 M ZnSO4,0.1 M Fe(NO3)3, 0.1 M CuSO4 in spot ratios of 8:12, 10:10, 12:8, 14:6, 16:4 & 18:2. It will be baked at a lower temperature of 300 to see if that reduced the flaking seen in past plates.
Green Team tested their plate of Cu and very dilute Zn. #1 was the lightest spot, it was light grey. #4 was brown, grey, the color settled in the middle. #5 was the darkest and looks most like the Cu control. They chose the spots that were highest on the negative DC tests, and lowest on the positive DC tests.
In the scans without Na2SO3, the peaks were in the same location as last week. Spot #4 peaked when a positive bias was applied. For these scans, spots 1 and 5 were significantly lower than the surrounding spots. When the bias was reversed, the peaks flipped, as they did last week. Next week they will spot different ratios of copper and diluted cobalt, one of the higher peaks (Spot #5) from the plate on 3/7.
Beckman Group 1 epoxied their plate (BHS-1-52) and are planning to test it next week. Regarding their new plate on aluminum iron (BHS-1-53), when spotting, the drops spread out from the pipettes and did not form coherent spots. They are still planning to bake it and test it next time. Group 2 is continuing their tests on bismuth-based plates. They made two plates (BHS-2-71A and BHS-2-71B) that tests various concentrations of bismuth and iron. Group 3 made many 0.04 M bismuth nitrate solutions in order to test their solubility in different solvents such as HNO3. This is because they do not have 2,4-pentadione nor glacial acetic acid, so they need to find some other way of dissolving the bismuth nitrate. Group 4 continued with their testing of the effect of UV treatment on the FTO plates. They epoxied thirty minute UV treated plate, but observed that it had been slightly damaged while in storage. They decided to UV treat a new plate for thirty minutes to spot it next week.
Week of 3/13-3/19
Beckman Group 1 started baking their plate (BHS-1-52). This plate had 0.03 M of iron standard and 0.03 M of Al(NO3)3. The ratios of Al: Fe are 4:1, 3:1, 2:1, and 1:1. They are also going to make another plate similar to BHS-1-52 but will increase the ratio of iron standard. Group 2 decided to add a little bit of HCl to their bismuth nitrate solution, specifically 2.0 M in order to help the solution dissolve faster. However, the bismuth just ended up sinking at the bottom of the volumetric flask. Group 3 had made a 0.03 M solution of bismuth nitrate last week. As per the advice that was given, Group 3 also added a little bit of HCl, in order to help the bismuth solution dissolve faster but got the same results as Group 2. Group 4 tested two of their plates, BHS-4-54A and BHS-4-57, which they had made a few weeks back. One of the plates was treated with the UV for 15 mins and the other was treated for 30 minutes.
Canyon Crest got through both of the plates they had ready to test (3 and 5), epoxied five more (6 through 10), and tested plate 6 as well. They also annealed three more (11 through 13), including the product of the evaporation/hot plate mini-experiment. They were also able to create two new plates, Ba:Cr:Sr and Fe(NO3)3:K2Cr2O7:KOCN (14 and 15). They had issues with the spotting template not lining up with the kit, so they made a new template which should hopefully result in less overlap between spots when testing future plates. Next week they hope to be able to test plates 7 through 10, epoxy 11 through 13, anneal 14 and 15, and perhaps make a new plate or two.
San Marino Green Team Tested their Cu and trace amounts of Zn plate from last week (99:1 and 999:1 Cu to Zn). Everything on the plate is different shades of grey but there doesn’t seem to be any predictable trend for why certain spots appear darker than others. Possible explanation could just be slight variations of how much volume is put down for each spot, though that seems unlikely to be responsible for such varying darkness differences.
When a positive bias was applied to the plate before scanning, the area of the plate without spots would peak, while the actual spots themselves were quite low. However, when negative bias was applied, the peaks were inverted so that the lowest peaks before were now the highest peaks. This means that in the copper reversed the current so that it would read a negative value. Overall, for every scan performed, the same spots peaked, usually the spots that contained the most copper. To test this hypothesis, they made a plate with only the highest and lowest amounts of copper to ensure that the data is accurate.
Blank Team Testing a plate with Ba because of good results on the SEAL database. They are using Mn to test it with new metals and see if it still has issues with flaking as it did last year. Both pure Ba(NO)2 and Fe(NO)3 spots were flaking, likely due to too high temp, since this is a brand new Fe solution. Ba and Zn formed Barium Sulfate precipitate (white spots) where the spots become more white/opaque with increasing Zn concentration. Some of the spots flaked or dissolved in the NaOH solution upon testing, which has all blue bars.
Idea for new plate: Paul explained that because the optical band gap acts as a “cutoff point” for light, only higher-energy wavelengths above the band gap are able to excite electrons. The band gap is also the amount of energy released when the electron falls back down to the valence level. We are interested in this energy, so we want larger band gaps. However, larger band gaps mean that more light is excluded. Thus, we want to maximize the size of the band gap without excluding too much light. A possible solution is to link a series of plates in succession, each with different materials that absorb different wavelengths. For example, violet on top, blue, green, and red on bottom. We need 1.23 eV to oxidize water. Thus, we need at least a 1.23 eV band gap. http://pubs.rsc.org/en/content/articlehtml/2009/cs/b719545c
Week of 3/6-3/12
Beckman Group 1 is spotting a plate with ratios of iron (III) nitrate to aluminum nitrate. Their plate, 1-52, will have four different ratios of iron (III) nitrate to aluminum nitrate—1:1, 1:2, 1:3, and 1:4, each ratio having their own section on their plate. Group 2 ran Plate 2-70 with 0.01 M bismuth nitrate, since 0.03 M was not soluble. Their ratios of bismuth nitrate to iron (III) nitrate are 1:3, 1:6, and 2:3, and the fourth section solely has the pure 0.01 M solution of bismuth nitrate. They also reran Plate 2-68 with both 0.03 M chromium (III) nitrate and 0.03 M zinc (II) nitrate, using three ratios—0:1, 1:1, and 1:0. Group spotted plate 3-62 with ratios of bismuth nitrate to iron standard, which are 1:0, 1:1, and 0:1. Group 4 ran plate 4-54, the plate treated by UV for 5 minutes, as part of their efforts to discover the effects of elongated UV treatment on the coffee ring effect.
Poly: After brushing up on some conceptual understanding of band gaps, electron excitation, and the solar spectrum, we’ve decided to try spotting two solutions on one plate in order to take advantage of the band gaps of multiple materials. We hope that the relatively low band gap of Cobalt will aid in capturing a wide spectrum of light while an additional layer of Iron will actually carry out water-splitting. We’re using 0.1 M solutions of Cobalt Acetate and Iron Nitrate (the same solution as last time). In addition, we’ve taken to drying our recently-washed plates with Nitrogen, and we’re cleaning two plates with plasma again this week to further explore effect of plasma etching.
The other group cleaned eight plates using the plasma cleaner at the Atwater lab. We then went to the Kavli Nanoscience Institute to observe what a real clean room fab looks like. We took back the plasma cleaned plates to the lab and dried them with nitrogen gas to remove any remaining/gathered dust. Finally, we spotted two plasma cleaned plates with 0.1 Iron Nitrate, two with 0.1 Manganese Sulfate, two with 0.1 Cobalt Acetate, and the last two with both the Manganese solution and the Cobalt solution.
Alverno: BASIC group made an MnSO4 and TiO2 plate to see how they react with each other. They also made an Iron plate along with a Strontium and Manganese plate. They tried running plates 180306ES-1 and 180223ES-1 but had issues with dark current. CO2 group explored different concentrations of Iron standard plates and in doing so noticed crystallization on the 0.4 M plates. They made another 0.4 M Iron plate and a 0.1 M Iron plate. DOLPHIN group used a Dremel to cut metal into pieces so that they can raise the printer to fit SEAL plates inside.
San Marino Green Team: Last week’s plate had nothing but noise. In the beginning of the year, they believed they had discovered a Cu spot that was a huge hit, so they continued to play with copper. The reasoning for this plate is simply to finish “The Copper Adventure”. From the plate made last week, there was actually a solid spike in Spot #1 (Cu:Zn in a 99:1 ratio). Rather than just noise as per the plate tested last week where everything was under 0.1, Spot #1 was reproducibly good, peaking up to 0.3 on the scale while many of the other spots hovered around 0.1. They expected to have bad results to finish off the Copper narrative, but instead found something interesting— though it was still blue, it is worth looking at if Zinc is a viable combination with Copper.
Blank Team noticed there is significant flaking for the iron control spot – Fe(NO3)3 after annealing. Other spots containing Fe did show some flaking, but not as much. As concentration of iron increases, the spots flake more and more. The reason for the significant flaking is probably because the Fe(NO3)3 solution used was old and cloudy. It could also be because the plate was baked at a 450 degrees C, which is higher than normal temperature.
When the plate was placed in the base solution, before applying voltage, significant flaking occurred, specifically on the “pure” MnCl2 spots and the iron control spots. Despite the flaking of the MnCl2 spots, much of the spot material remained attached to the plate in a deformed state (in pieces instead of a round circle). The iron control spots, which had already significantly flaked after baking before placing it in the base solution, completely flaked away after being placed in the base solution. For the first scan, the dark current is around 0.5V, and applied a current of -0.002V. MnCl2 had a the highest bar (0.2V) out of all the spots. Second scan was performed with -0.002 V applied; dark current remained steady and dropped after the second scan. The third scan applied -0.005 V with dropping dark current; results are overall the same. Scan four has the same result despite the positive applied potential 0.005. Overall, given the four scans, there are no conclusive results.
They are planning to use barium for the next plate because they don’t have any cadmium. They will mix the barium salt solution with a manganese salt solution and repeat some spots containing iron using a newer solution. Since the vanadate tended to react to form new materials, they will not test it for this new plate. 10 microliters per spot of:
0.2 M MnCl2, 0.1 M Ba(NO3)2, 0.1 M ZnSO4, 0.1 M Fe(NO3)3
Ba(NO3)2 + MnCl2 (7.5 : 2.5); (5 : 5); (2.5 : 7.5)
Ba(NO3)2 + Fe(NO3)3 (7.5 : 2.5); (5 : 5); (2.5 : 7.5)
Ba(NO3)2 + ZnSO4 (7.5 : 2.5); (5 : 5); (2.5 : 7.5)
MnCl2 + Fe(NO3)3 (7.5 : 2.5); (5 : 5); (2.5 : 7.5)
MnCl2 + ZnSO4 (7.5 : 2.5); (5 : 5); (2.5 : 7.5)
ZnSO4 + Fe(NO3)3 (7.5 : 2.5); (5 : 5); (2.5 : 7.5)
Week of 2/27-3/5
Beckman worked on testing their plates this week in a 50.0 mL of sulfite solution and 50.0 mL of NaOH solution. Group 1 started testing their plate (BHS-1-51). This plate had iron standard and the different ratios of 0.03 M of Cd(NO3)2 and Cu(NO3)3. The ratios of Cu:Cd is 5:1, 4:1, 3:1, and 2:1. Group 2 made an error with epoxy on one of their plates and will start testing next week. They also started epoxying another one of their plates (BHS-2-70). Group 3 started testing two of their plates (BHS-3-60) and (BHS-3-58). BHS-3-60 is a plate that is testing 0.03 M solution of iron standard and Al(NO3)3 with ratios of 2:1, 1:2, and 1:1. The BHS-3-58 plate is testing a 0.03 M of iron standard, Cd(NO3)2, Cu(NO3)3, and Al(NO3)3. The ratios of Al:Cd:Cr for this plate is 1:1:1, 1:3:1, 3:1:1, and 1:1:3. Group 4 will test their plate next week.
Canyon Crest did a short test of air-drying vs hotplate evaporation of spots (of Sr:Ba:Zn) and found that air-drying reduced the coffee ring effect. They also tested two more plates, CrCl3:K2Cr2O7:CoCl2 and K2Cr2O7:NH4VO3:KMnO4. They saw very good results in a spot with roughly equal proportions Co and Cr (which was promisingly surrounded by a gradient of high readings). One of the plates to be tested next week contains a spot similar to this so they will use that as an opportunity to confirm the result.
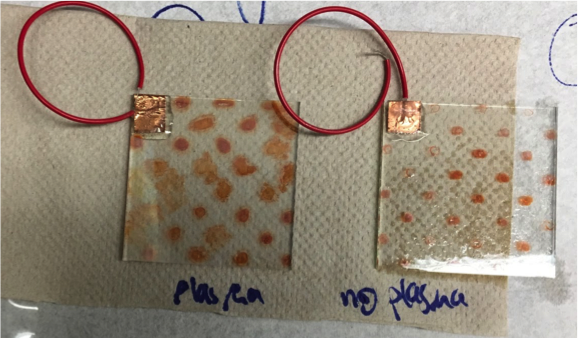
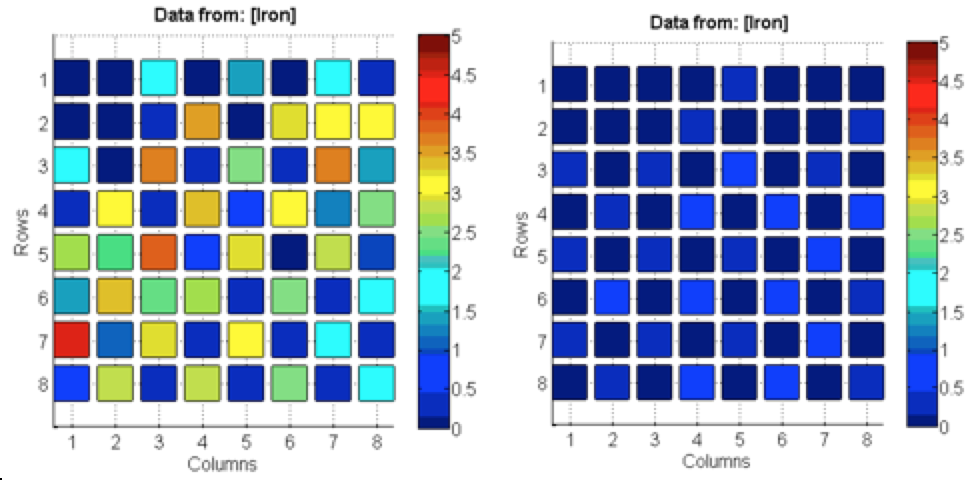
Poly finished epoxying plates of Fe(NO3)3. Tested both plates of Fe(NO3)3. One was plasma etch treated for thirty seconds before spotted. The other plate was treated normally → rinsed with acetone and isopropyl alcohol and deionized water. The Fe(NO3)3 plate treated with plasma had significantly better results than the non-treated plate, possibly due to the larger and less faded spots seen on the treated plate in comparison to the coffee-ring effect on the non-treated plate. See picture below
The data on the left was cleaned with an oxygen plasma and the data on the right was the control. Although these results show that there was success with the plasma cleaner, the results for the control were questionable given that they were unusually unsuccessful. Poly also looked for salts to layer with the iron and found manganese and cobalt had good band gaps. They made a 0.1 M MnSO4 solution, will make the 0.1 M cobalt solution next time.
San Marino Green Team observed last week that for spots with a higher concentration of Cu, the addition of Na2SO3 increased the relative size of the peak. They tested a new plate this week, baked at 400℉. (1) 100% Cu, Uniform dark gray; (2) 0:100 Cu:Ni, uniform pale gray; (3) 50:50 Cu:Ni, uniform dark brown; (4) 75:25 Cu:Ni, Dark yellow brown; (5) 25:75 Cu:Ni, lighter yellow brown; (6) 90:10 Cu:Ni, looks very similar to (1), uniform dark gray.
While at first, they observed that certain peaks were present in the graphs, the low overall voltage led them to the conclusion that this plate was mostly “noise”. The variation in the dark current was greater than or equal to the height of these peaks, meaning that we could not comment with any certainty on the performance of individual spots or the effect of adding Na2SO3. For next week’s plate, they have decided to continue the narrative with copper. They will once again revisit the 1:99 and 1:999 ratios, with Cu as the base material and Zn and Co as the trace materials. They will also test this plate in Na2SO3.
Blank team made a new plate (10 microliters per spot, most spots dried out before baking in kiln). Solutions used were 0.10 M NH4VO3, 0.10 M ZnSO4, 0.10 M Fe(NO3)3, 0.20 M MnCl2. Made spots with ZnSO4 + NH4VO3 (2.5:7.5), (5:5), (7.5:2.5); Fe(NO3)3 + NH4VO3 (2.5:7.5), (5:5), (7.5:2.5); MnCl2 + NH4VO3 (2.5:7.5), (5:5), (7.5:2.5); 0.1 M Fe(NO3)3; 0.2 MMnCl2; 0.1 M ZnSO4.
Week of 2/20-2/26
In order to be able to run more plates and become more efficient in the long run, Beckman decided to install the SEAL Kit Software onto another computer. Because it took time, it was too late to run any plates this week. After researching and looking through previous lab notebook reports, Group 1 concluded that there had been the most success using iron nitrate and aluminum nitrate so far. Therefore, they decided to prepare a plate with different ratios of aluminum nitrate to iron nitrate. Group 2: saw there was success using bismuth from PCC’s work. Therefore they decided to retry bismuth with a lower concentration (0.01 M) in order to achieve greater solubility. Then, they spotted a plate using different ratios of bismuth nitrate and iron nitrate. Groups 3 and 4 installed the SEAL Kit Software onto a Windows 10 laptop and set the SEAL Kit up to confirm that it worked. They also spotted a plate with 0.03 M Fe(NO3)3 and then UV treated the plate for thirty minutes as part of the research on the effects of different time intervals of UV treatment.
Canyon Crest Academy was able to spot two more plates and kiln and epoxy several others. However, more interesting is what they failed to do: make a plate of iron, tin, and lead. They attempted it three times and discovered issues with all three solutions. The first mistake was using tin (II) chloride (SnCl2)which was past its shelf life. This particular bottle of tin had been prepared in 2006 and had apparently transformed into stannic chloride (SnCl4) via exposure to air. Second, there were some precipitation issues with a beaker of iron sulfate mislabeled as iron nitrate (leading them to believe it was soluble in lead). Finally, they didn’t take into account the limited solubility of the lead chloride solution made (the solubility of lead which is 10.8 g/L; the attempted solution was 27.8 g/L). These issues were resolved quickly though and they made two new plates.
San Marino’s Blank Team: Last week’s plate had severe coffee ringing effect, but it is unlikely that it will actually affect test results. The greater the concentration of copper/nickel, the darker the oxides are. 100% Sodium Metavanadate is white in color; 100% Ammonium metavanadate is white-yellow in color.
Potential materials stable above/below water oxidation line:
- Manganese vanadate
- Manganese oxide
- Cadmium oxide
- Barium oxide
Testing: dark current = 0.1-0.3V and applied voltage = 0.07V. The current increases from left to right, with the 75% nickel/copper compounds resulting in the highest current. The highest peak was only ~0.2V, and the differences in height were not dramatic (within 0.1v). The purported sodium metavanadate and ammonium metavanadate spots washed away (as expected; they should be water soluble). This is similar to what occurred last year when sodium metavanadate washed away from one of our plates. Overall, no significant results.
Made new plate (total volume each spot is 10 microliters):
ZnSO4 + NH4VO3 (2.5:7.5), (5:5), (7.5:2.5)
Fe(NO3)3 + NH4VO3 (2.5:7.5), (5:5), (7.5:2.5)
Fe(NO3)3 control
Week of 2/13-2/19
Beckman Group 1 created a sodium sulfite solution for testing. Because the epoxy has proven to be weaker when being put into the testing solution after five to ten minutes of drying, they are waiting for 45 minutes for it to dry before testing. Group 2 is testing a plate with chromium nitrate with iron standard that they made previously. They also plan to test a plate with zinc nitrate, chromium nitrate, and iron standard afterwards. Groups 3 & 4 collaborated in continuing to test the effect of varying lengths of UV treatment of the FTO plates. They have prepared the plates of 5 min, 10 min, 30 min and 60 min and they are trying to test the plates. Today they are running the 5 minute plate. Last week, they realized that their iron nitrate solution was old and looked cloudy, which they predicted may skew their results. Today, they recreated the solution to make the same 5 minute UV-treated FTO plate to compare it with the old 5 minute UV-treated FTO plate to see if there is any difference.
Alverno has updates from their 4 teams. Acid: Made 4 plates of Nickel Nitrate and Nickel Nitrate Titanium Dioxide to test this week. Basic: Have 4 plates to test, all of which plates have been made with two different metals at the concentrations 0/100, 25/75, 50/50, 25/75, and 0/100. There are a total of 14 spots on each plate. The metals were chosen at random in hopes of stumbling upon a combination that works very effectively. CO2: Figured out a relatively good concentration of TiO2 and the acid group is going to proceed and use that concentration with their future studies. The CO2 group on the other hand had abandoned the idea of TiO2 in their plates, and started randomly picking metal combos from the periodic table (with certain selections). The SEAL kit has been malfunctioning so they are holding back on making more than their existing 10 plates until that is resolved. DOLPHIN: They have cut the printer in half so that they can raise it’s height for the seal plates and they will reattach it with a spacer.
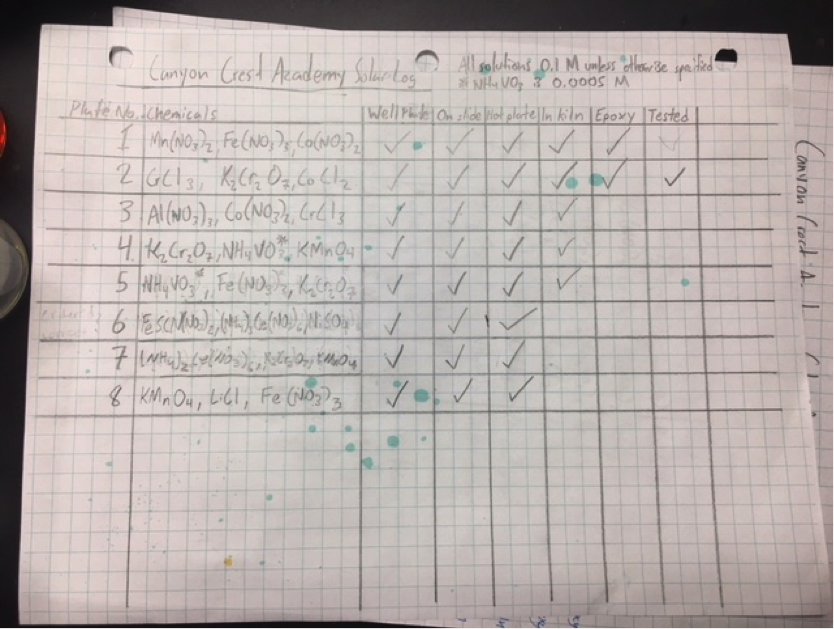
Canyon Crest Academy has gotten organized and created a sheet listing out all of the plates and materials made so far (to avoid testing duplicates). They were also able to create an two additional interesting plates using Iron Ferrocyanide, Ammonium Cerium Nitrate and the potassium chemicals Potassium Dichromate and Potassium Permanganate. In preparing these plates, the metal cations in the solution were not homogenized in the solution: causing the hot-plated slides to have some spots with the coffee ring effect or small pieces of precipitated matter. They expect this to impede getting “good data” since the spots will not be tested as completely as they would otherwise. To avoid the rapid evaporation in future, they will set the hotplates on lower temperature settings and keep a keener eye on the homogeneousness of solutions. Because of their surplus of prepared and epoxied plates, the next meeting will be dedicated to testing existing plates.
Weeks of 1/23-2/12
It’s been a slow few weeks so I combined the updates into one post.
Poly tested two MnCaO and two MnVO plates but didn’t report on the results (not good?). They also wanted to try a different method of pre-cleaning plates to allow for better results, so they went to the Atwater lab and used their plasma cleaner for 30 seconds. This should get rid of any extra debris and material on the plates. They spotted Fe(NO3)3 to test as a control.
Beckman Group 1 re-epoxyed their plate from last week, whose epoxy binding had come apart in the sodium sulfite solution after they let it dry for well over five minutes. They will let the epoxy sit for about an hour before they test it again. They are also baking the plate that they previously spotted. Group 2 is testing a plate with chromium (III) nitrate. They are also making a new plate with chromium and zinc with a ratio of 1:1. Group 3 made a new 0.03 M iron nitrate solution since they were running low. They also set up last week’s plate for testing, and will test it after the teams that are in line for testing. Group 4 has discovered several inconsistencies that may have played a role in how their plates turned out. They had set up a plate last week for testing this week – which was UV-tested for five minutes – so they planned to test the plate today. However, the five-minute and one-hour treated plates had much better consistency in their spots than the ten-minute plate; they switched to a new micropipette for the ten minute-plate with a thinner tip, which may have resulted in discrepancies. Moreover, the iron (III) nitrate solution that they used for the four plates has become cloudy, so they will have to remake the solution and UV treat a new batch of plates starting next week, using both the new solution and the new micropipette. Despite this, they will still follow through with testing the plates they already have, including the thirty-minute plate, which has air dried but will needs to be fired.
San Marino Blank Team Tested the new nickel-copper plate. Spots were clean and had minimal coffee ring effect. Iron spots are slightly flaking. Overall, the least flaking of all the plates done so far! Plate did not flake much when placed in KOH solution. All tests: every area (spotted and unspotted) had blue peaks, with exception of the two Cu control spots. Results are inconclusive (perhaps except for the flat Cu spot)
Made new plate (total volume each spot is 10 microliters):
0.1 M Cu(NO3)2 + 0.1M NH4VO3 (2.5:7.5) – Turned bright yellow, (5:5), (7.5:2.5)
0.1 M Ni(NO3)2 + 0.1 M NH4VO3 (2.5:7.5), (5:5), (7.5:2.5)
0.1 M Fe(NO3)3 control
0.05 M NaVO3 (sodium metavanadate)
0.1 M NH4VO3 (ammonium metavanadate)
Green Team tested the plate made on 1/22, got no significant peaks, probably due to the low temperature (~375℃) at which it was fired. Remade the plate but got deep coffee rings this time with not much substance in the middle. Worrisome since it could mean that the light is not actually hitting the particles and only faded parts of the particles. This might mean that the testing on this plate was flawed. Results were as usual— all blue with negligible difference even when the concentration of a material multiplied by ten. However, the substances are in a 1:999 ratio and a 1:99 ratio, so it seems like these materials aren’t actually contributing at all. The plan for next week is to plate a control of copper and iron to see how hydrogen peroxide layered over the spots influences the results and also see if the materials are good light absorbers or not.
Earlier in the week, they plated 0.10M Fe(NO3)3 and 0.10M Cu(NO3)2 in a checkerboard pattern as a control. They aimed to observe the effect of adding Na2SO3 to our NaOH testing solution. The plate reached a temperature of 500℃ and the Cu spots were solid, dull, and black. The Fe spots had a faint red/brown coffee ring, with shattered metallic bits in the center. These bits flaked off once the plate was inserted into the NaOH solution. Initially tested the control plate in the regular NaOH solution. With no bias applied, Scan #1 displayed very low Cu spots, while the Fe peaks and background dark current were at roughly the same level. After applying a voltage of 0.05V, similar results were observed. Subsequently, applied a voltage of -0.05V. This time, the Cu spot peaked, with the dark current on the same level as the low Fe. With a voltage of -0.1V, the results were the same, except the dark current increased slightly.
They then replaced the NaOH solution and added 0.1 mol of Na2SO3 to the 100 mL of NaOH solution. With no bias applied, the Fe peaked again. The Cu and dark current remained at the same level. With 0.05V applied, the dark current increased slightly. Just before -0.05V could be applied, the voltmeter ran out of battery and had to be replaced. The dark current briefly read 0.1. At -0.1V, the dark current was -0.3. The Cu spots peaked this time, with the dark current significantly higher than the low Fe spots.
With the addition of Na2SO3, the peaks seemed to decrease in height, possible as a result of the plate spending a long time in the solution. With a higher applied voltage, the peaks seemed to return to normal. When applied voltage was reversed, it was that the peaks flipped, with Cu now higher than Fe. Whether or not they expected this outcome depends on how the SEAL program interprets negative values…
Week of 1/16-1/22
Beckman‘s Group 1 fired the Cr(NO3)3, Cd(NO3)2, plate from last week and treated a plate with UV to prepare for next week’s endeavors. Group 2 made a solution of 0.03M of bismuth nitrate, spotted their plates and plan to let it dry over the weekend and fire it before next Friday’s meeting for testing. Group 3 spotted a UV treated plate with 0.1M of Fe(NO3)3 and Al(NO3)2 using three different ratios of 2:1, 1:2, and 1:1. They are planning to fire the FTO plate and test it next week. Group 4 has been continuing to test the effects of varying length of exposure to UV treatment on the FTO plates. Today, they etched a new, clean FTO plate and UV treated it for exactly five minutes. This plate that was treated for five minutes was spotted with 0.03M Fe(NO3)3 solution to be fired in the oven. They also did the same for another plate etched with UV for sixty minutes.
Canyon Crest was able to create 5 more well plates of materials this week including Al:Co:Cr which is pictured below and has an interesting greyscale arising from the Chromium solution in different concentrations in the other, transparent, solutions. They also made a very interesting experimental plate consisting of 0.0005M NH4VO3; 0.1M Fe(NO3)3; 0.1M K2Cr2O7. They were able to create some nice looking color gradients (that are also easier to spot on the well plate when arranging the concentrations).
*Helpful tip from Canyon Crest: find the solubility of a solution before you prepare it- either from the bottle or a website like PubChem. They had a failed attempt at making BiNO3 solution trying to dissolve in Nitric Acid, Acetone, and Glycol while on a stirring hotplate – as the bottle instructed. However, the bismuth always precipitated down to the bottom of the beaker and individual particles were easily seen.
Crescenta Valley Team 2 attempted to spot Fe(NO3)3 on a plate last week that had been cleaned with the Tesla coil but the spots spread out. This week they turned to glycerin as a solution, finding a test solution of Fe(NO3)3 and glycerin more or less held together. During the meeting they worked on a new solution of iron nitrate and glycerin in a 1:1 ratio but had some difficulty measuring out the glycerin as it was viscous. They weighed it (instead of pouring it), knowing its density, and created a solution with which they can spot future plates that have been cleaned with the Tesla coil
San Marino Blank Team tested their last plate made in 2017. Flaking after baking is evident. Applied voltage of 0.05V and had dark current around 0.3V, but the results were all in the dark blue range. Reason why there are not so good results is because severe flaking during baking (temperature exceeded 500 degrees as evidenced by silvery powder within iron rings). This is true especially for Cu spots, which are very faint, probably not enough to make the “incident” from last week happen again. Next time they plan to spot a new plate using iron/copper controls. Iron is the “primary” control, copper control exists to see if they can reproduce the “incident” from last week. Main focus for this new plate is Ni and Cu: 100% Ni; 75% Ni, 25% Cu; 50% Ni, 50% Cu; 25% Ni, 75% Cu; 90% Ni, 10% Cu.