SoCal Infantry
Welcome to the Southern California SEAL infantry! SoCal SEAL students check here for important information regarding the SEAL program at Caltech
Important dates for 2019-2020 school year:
SEAL Kickoff: October 5, 2019 10am-12pm
SEAL Con XI: May 9, 2020 10am-2pm
2019-20 SEAL Roster
Every member of a SoCal SEAL team needs to sign-up on the roster. This ensures you will receive communications about upcoming events and helps us keep track of our membership. This also is how we make certificates for SEAL Con!
SEAL Permission Slip
All SoCal SEAL students need to fill out this permission slip and return it back to Kitty Cahalan either via email (kcahalan@caltech.edu) or in person through their mentor. Students under 18 must have the form signed by a parent or guardian.



Archives of SoCal SEAL Blog
Click on a blog post to view and add comments
Weeks of 2/13-2/26
Double post this time- last week only had a couple updates, and it looks like only the same few teams posted again this week. The blog will only be useful if everyone is consistent with their weekly posting, so please set reminders to update your google drive doc every week (and thank you to those teams who do post consistently)!
Poly attempted HARPOON one more time, but ran into a new hurdle: the power supply wasn’t working properly. There is also a battery pack power supply that should work with a fresh set of AAs, so you can try that next time. The Poly team is also gearing up to do Raman Spectroscopy on their bismuth nitrate material. They learned all about Stokes, Anti-Stokes and Raleigh spectroscopy and have decided to use Stokes for this experiment. If you want to learn about this method, reach out to the Poly team!
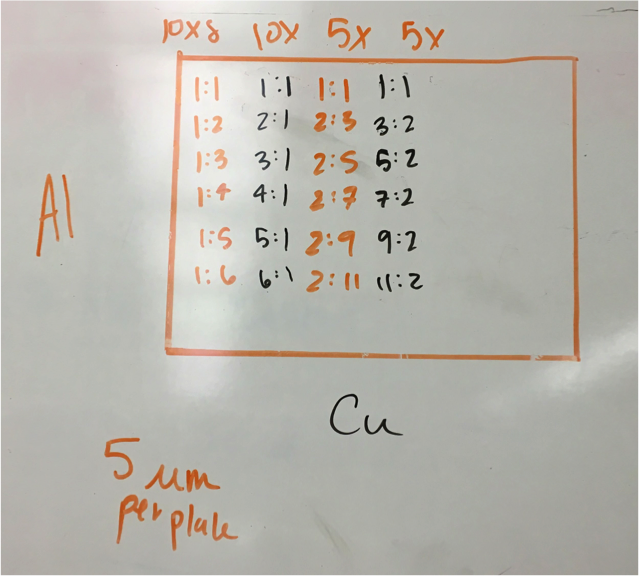
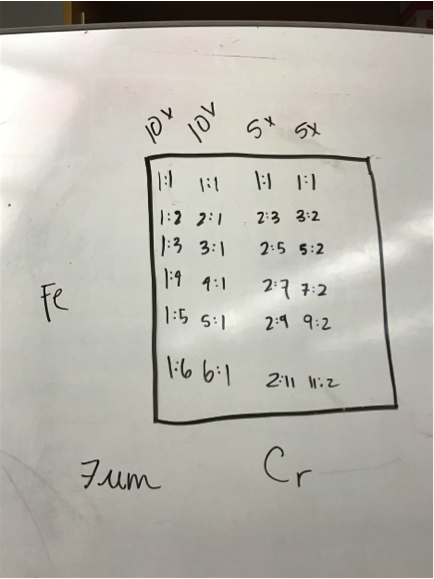
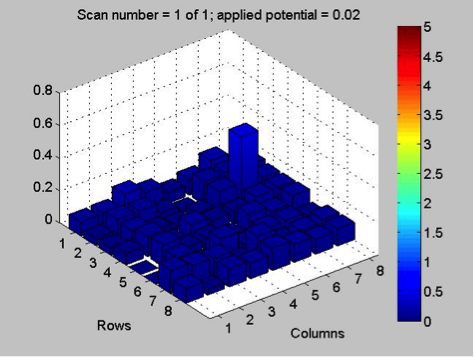
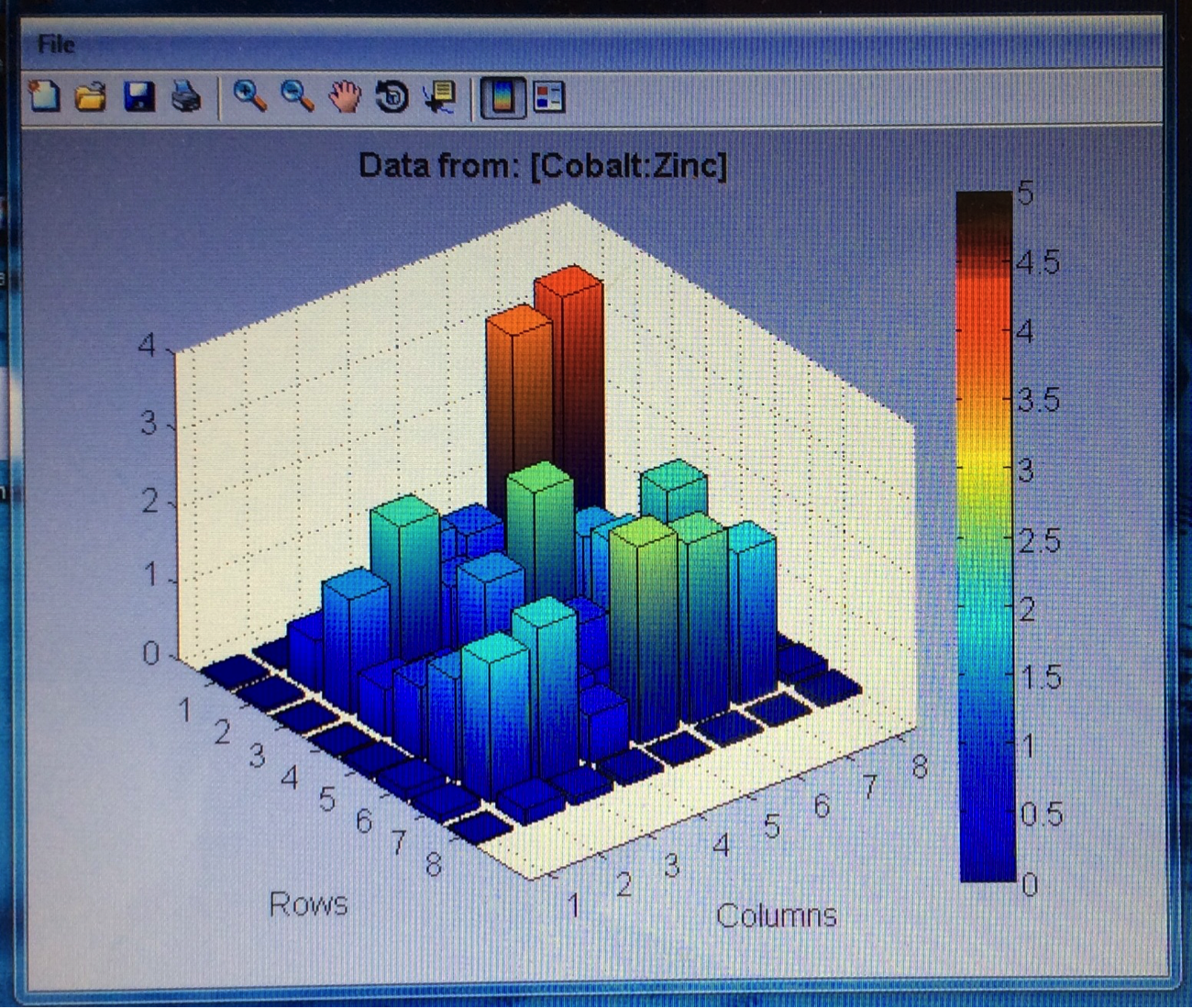
Mayfield’s Team PEAK added a second layer to their split Co/Fe-Zn plate (6:1 ratio). They are testing the hypothesis that applying multiple layers of solution onto the same spot will increase the success of a plate and aid in lessening the negative effects of contamination. The first half of the plate possesses two layers, while the second contains three. Team RAM finally got their sodium metavanadate to make a new vanadium solution. They made a plate with BiVNi in a ratio of 8:1:1, 8:1:5, 8:1:5. The results were in the light blue region, so they are continuing in that vein making a BiVNi plate with ratios of 8:1:1, 8:1:13, 8:1:16. Team SEA has decided to test various ratios of 2 metal combinations in more depth and so are using the following scheme below. The plan to eventually test Ti, Fe, Cr, Mg, Al, Cu all this way.
Week of 2/6-2/12
Sorry for the late post this week! Starting off Crescenta Valley made another plate using just 0.1 M BiVO4 and another using 0.1 M BiVO4 layered with 0.05 M Co, and BiVO4 layered with .05 M Ni and .05 M Fe in a 1:1 ratio. They want to test the effects of Co on BiVO4 compared to Ni:Fe. They also made a solution of 0.05 M Fe(NO3)3 using ethyl alcohol instead of water. Their mentor suggested that this might help with the coffee ring effect; they will spot two solutions, one with water and one with ethyl alcohol, and see if the coffee ring effect is diminished.
Beckman HS’s Group 1 researched to find future work: band gaps of different metals. They also UV treated a new plate of 0.04 Iron (III) Nitrate because they had positive results last time they tested it. They wish to continue to test the effect of UV treatment and find optimal metal oxides/specific molarities that work well with UV treatment. Group 2 tested a UV treated plate with ratio of Nickel, Iron, Aluminum (1:3:1; 0.03:0.09:0.03). They are planning to compare with a non-UV treated plate (same ratio). Since Nickel is a less than optimal photoanode, they researched other metals and decided to replace Nickel with Copper next time. Group 3 finished spotting the layered plate they started last week (0.04M Cobalt(II) Nitrate over 0.04M Iron(III) Nitrate) by adding the Cobalt Nitrate layer on top. They fired the mixture plate (mixture of 0.04M Cobalt Nitrate and 0.04M Iron Nitrate). Their goal is to compare the methods of mixing two metals and layering them because there were various groups at CCI with positive results that used the layering method. They re-tested the Cu-Ni-Fe plate because of dark current issues they had last time they ran it. Group 4 epoxied 0.03M Copper (II) Nitrate plate and ran it. Group 1 tested 0.04 Copper (II) Nitrate in the past, so they will be comparing the results with each other and determine which molarity works best with Copper.
San Marino Red team decided to use Ni(NO3)2, MnCl2, and CuSO4 to test these materials for flaking and/or corrosion. They found very little flaking—a good sign! Their hypothesis for this finding is that the absence of significant flaking was due to the low concentration of the spot solutions (only 0.05 M) and leaving it on the hot plate for an 30 extra minutes. The photoactivity of the spots was not as high probably because the plate was not baked and that the spot solutions had a lower concentration than usual. The spots changed color as the plate was being tested (as the current was flowing)—–Not a good sign! This could mean corrosion or (a) chemical reaction(s) may be occurring. For next week’s meeting, they plan on testing this same plate after it is baked in the kiln.
The San Marino Wand team decided to continue with the hypothesis that a good catalyst would be a good light absorber. However, this time, knowing to focus on oxygen evolution, they looked towards compounds such as BaNiO3 and Ni2Se3 and NiFeO, and NiMO on the OER side. On the light absorber side, Silicon. They ended up plating: Cu, Co, Iron, and Nickel, on a base of ammonium vanadate. Testing the plate they found nickel worked best so they made a new plate with just ammonium vanadate and different ratios of nickel.
Mayfield’s PEAK team retested the plate of FeCoZn from last week with new wire epoxied in a different corner. While this did decrease activity of the very active spot next to the old wire, the spot was still quite active compared to the rest of the plate. Their next experiment is to test efficiency of multiple layers compared to a single layer so they made plates 21 (FeZn with multiple layers) and 22 (CoZn with multiple layers). Team SEA got marginal results from a 4:7 ratio of their colored to non-colored metals. Retesting the old plate didn’t yield good results, but remaking the materials the result was reproducible. They will be looking at 1:8 and 5:8 ratios next. Team RAM has disappointing results from their Bismuth, Vanadium, and Tungsten and Bismuth, Vanadium, and Nickel plates. Perhaps a new solution of vanadium will help. If any other teams have tips for using ammonium metavanadate since it is not very soluble, please comment!
Concordia attempted three different tests. First, tried to test CONa3 which was bismuth nitrate and ammonium vanadate with the concentration ratio ranging from 1:1 to 1:1.7, the higher being bismuth nitrate. Unfortunately the epoxy fell out when they put it in the sodium sulfite electrolyte solution because it wasn’t dry yet. Second, they used the tesla coil gun and shocked the CON8 plate for testing for next week. The spots changed from a yellow to a reddish brown color. Third, they tried to do our own UV/Ozone treatment. However, due to the lack of appropriate materials in the lab, they were unable to do the testing. They will be using the set-up at Beckman HS next week.
Finally Franklin team 4 is testing some new materials- Ni Cr Y W. The spots were spreading quite a bit, and even adding glycerol didn’t solve the issue. They made two plates with these materials and fired them both to test with the HARPOON kit next time. Team 5 was having trouble seeing any oxygen produced when testing their plate with HARPOON, even at a higher voltage. They plan to add 10% glycerol to their spots next time to see if that will help.
Thanks for all the great updates everyone!
Week of 1/30-2/5
This week we’re back in the swing of things. Not everyone posted updates so please try to remember to record your progress every week on google drive. It’ll help us all stay connected and get better results by the end of the year.
First update is from Oakwood School. They debriefed on the CCI Solar Annual Meeting and decided that they will continue to test plates (though still working on the parameters for that research), so they started by making an iron nitrate plate at the last meeting. They will also focus on writing up a report on how to build and use their UV Ozone cleaner. That way all the other teams will be able to follow the plans to build their own device and achieve more uniform spotting. If you’re interested in learning more about the UV ozone cleaner, reach out to Oakwood in the comments section and let them know any questions you have that they should address in their report.
Poly reviewed what they learned at the Annual Meeting as well. They had a discussion about the Juice from Juice project, and then retrieved new batteries to improve the UV light conditions for their HARPOON testing. They then did calculations to prepare a solution of a HARPOON standard (Ni/Fe/Co) used by all the other HARPOON sites, and cleaned plates for next time.
San Marino had a conversation on the Annual Meeting too and decided that they want to try using a UV ozone cleaner too if possible. They also saw materials that other groups are working with and are willing to potentially revisit bismuth (despite previous experiences with the material flaking) and cobalt. They particularly were impressed with a result shown of CoZn that was in the red with very high current.
At Mayfield, team PEAK contacted Mike McDonald (MIT) who spoke at the conference about his experience studying variation in SEAL results and received an excel file where they can input various current values for the same material and determine the standard deviation of the current. They also tested a CoZnFe plate and found another promising material (similar to their one CoZn material). They plan to epoxy a new wire to the opposite side of the plate to verify that the material being close to the bias potential didn’t affect the result.
Team RAM made two new plates: plate 11 contains Bismuth, Vanadium, and Tungsten (with the ratio of 8:1:1, 8:1:5, and 8:1:8), and plate 12 contains Bismuth, Vanadium, and Nickel, with the same ratio of plate 11. They used ammonium metavanadate for these plates that wasn’t very soluble so the ratios will likely be off. Hopefully next week the sodium metavanadate will be available to remake the materials and compare efficacy. Team SEA continued making plates with their colored and non-colored salts in more ratios (1:8, 4:7, 5:8).
Week of 1/23-1/29
Everyone was prepping for the Annual Meeting so no updates from this week.
I hope everyone who was able to attend had a good time. All the posters were FABULOUS and I loved seeing the great work each of you has been doing. Very impressive. You guys did a wonderful job presenting and I heard many compliments on your work. Remember to take to heart the advice you got from your fellow scientists at the meeting and think of ways to start on some of their suggested experiments. Incorporating new experiments and directions to your research plan will only improve the final results for SEAL Con on May 13!
Now back to regular updates for next week please! And keep those conversations you had last weekend going in the comments…
Week of 1/16-1/22
Most teams have been busy working on their posters for the Annual Meeting. Remember to leave enough tie for printing and to bring your poster with you! Alverno is hoping to conduct a study where they test other teams’ plates with their SEAL kit at the Annual Meeting. If you want to be part of this Interlab study, bring your plates with you to the meeting!
Crescenta Valley tested the two plates from last week (NiCl2, ZnCl2, CoCl2), but had negative results- dark blue for both. They made several more plates, but we haven’t been able to get them annealed yet, so will finish testing them next week. One plate had .1 M BiVO4 with .1 M CoCl2 layered on top; another combined CoCl2, ZnCl2, NiCl2 in differing ratios (Co:Ni 1:1, Ni:Zn 1:1, Co:Zn 1:1), with four spots of Fe(NO3)3 as a standard; last plate had spots combining Ni:Fe:Co in a 1:2:2 ratio.
Mayfield ‘s team RAM made plate 9 (CuW) with the ratio of 10:6 and plate 10 (AlW) 10:6. Team SEA has noticed a trend with their colored & non-colored metal combinations: a higher ratio of non-colored to colored metal seems to produce more active materials. Their most active spot so far was iron tungsten in a 4:7 ratio.
Franklin’s team “Versace” has posted the combinations of metals used on their first 3 plates. The metals they are testing are BiVO4, Co (II) acetate, Fe (III) nitrate, Ni (III) nitrate, and WO2Cl2. They will be using the HARPOON kit I believe, and after results are posted, I will point out specific ratios that had above average results.
Keep up the great work and see you soon!
Weeks of 12/19-1/15
Hopefully everyone had a fun and restful winter break! Most teams have had a first meeting back by now, so I’m sharing updates from the past two weeks. In addition to tackling research again, most teams are hard at work preparing posters for the CCI Solar Annual Meeting on Jan 28 in Newport Beach. Congratulations to all the teams who were invited to participate based on their contributions to the Drive docs and blog comments! Keep up the great work and remember to check the comments for questions asked of your team that you can answer!
Alverno started up the new year with a plan to switch to an acidic electrolyte instead of the sodium hydroxide electrolyte. They prepared nickel nitrate plates for testing in both acidic and basic conditions. They used titanium dioxide paste as a “protective layer” at the base of the spots to protect from corrosion in hydrochloric acid. Looking forward to hearing more about this!
Beckman’s Group 1 found upon testing their .04 molarity of Iron(III) nitrate plate that it had a high concentration. Therefore, they decided to remake the .04 molarity of Iron (III) nitrate test plate. They made the whole solution again and spotted the solution onto the new plate. Group 2 ran two plates with the same materials (one UV treated and one non UV treated). The plate consisted of three different solutions: 0.03 M Ni (NO3)3, 0.03 M Al (NO3)3, and 0.03 M Fe(NO3)3. They also made a new sulfite electrolyte solution, Na2SO3, with remaining time. Group 3 tested the BHS 3-47 plate which consisted of different ratios (of?). They analyzed their results, finding out the averages and looking over the ratios. Group 4 tested the leftover Fe(NO3)3 plate from before winter break. While waiting for the computer, they made a new solution of 15 mL of .02 NaOH, Na2SO3, and different sodium sulfites. This solution was to be put in a big bowl for testing future plates. They also researched about crystal structures and hematites online.
Crescenta Valley made two plates using differing ratios of NiCl2, ZnCl2, and CoCl2. They used iron as a standard on four spots in the corner, and then spotted in a checkerboard pattern using the other three solutions (ratios of Ni:Zn:Co in 1:1:1, 1:1:2, 1:2:1, and 2:1:1 on one plate, ratios of Zn:Co in 1:1 on another). They also were able to print the 3D printed holder from Fairmont State (hooray! finally!) They haven’t been able to test it yet because of the assembly required, but hopefully it will be ready to test by next week.
Mayfield had a glitch with their computer log-in and were unable to collect data at the last meeting, but both SEA and RAM teams have several plates ready to test for next time. Team PEAK (formerly KEN) is doing more research on applying a negative bias voltage and what that means in terms of data collected with the SEAL kit.
Can’t wait to see all your posters in a couple weeks. Keep up the great work and remember to post weekly, comment and ask questions!
Week of 12/12-12/18
Most teams are on vacation this week, but here are a couple quick updates from Poly, Beckman, and San Marino:
Beckman’s Group 1 tested the UV-treated plate spotted with solutions of 0.04M Cu(NO3)2, which had relatively poor results compared with the UV-treated 0.04M Fe(NO3)3 plate from last week. They will have two members come to school during winter break in order to continue firing and testing the remaining UV-treated plate spotted with 0.04M of Ni(NO3)2. They will compare the results of different metal oxides to determine which chemical produces the best results, and then they will take the best chemical and alter its molarity for future experiments. Group 2 worked on spotting a plate with a combination of Al(NO3)3, Ni(NO3)2, and Fe(NO3)3, (all of 0.03M) in a ratio of 1:1:3, respectively. They decided to use aluminum and nickel based on their research on these metals from last week: aluminum would allow for more adherence, while nickel allowed for more uniformity due to smaller particle sizes. Group 3 compiled and analyzed their results from last week’s testing of their plate, which was spotted with 0.1M of Fe(NO3)3, Cu(NO3)2, and Ni(NO3)2 in different ratios. They are planning to utilize the newly 3D printed template to accurately spot more plates of the same ratio. Group 4 had planned to test their non-UV treated plate spotted with 0.03M Fe(NO3)3 in a sulfite bath. However, their epoxy did not dry in time for them to be able to test their plate during today’s meeting. They plan on continuing next week.
San Marino’s Red Team attempted to test the plates that were prepared, drop casted, and baked in the kiln last week. (See pictures and description from December 5) Unfortunately, however, they were unable to find the Cu + Ni plate and were only able to test the Mn plate. However, once the Mn plate was submerged into the NaOH and a dark current was applied, some of the deposited metal oxides flaked off from the plate, including one entire spot of metal oxide. Flaking has been occurring with most MnCl2 materials. It is interesting to note, however, that the spots drop casted from only MnCl2 and the spots drop casted from MnCl2 + Ni(NO3)2 seemed not to flake off as much than as the spots drop casted from Copper (II) Sulfate + MnCl2 and the spots drop casted from Cobalt (II) Chloride + MnCl2, both of which almost completely flaked off.
Poly finally tested their bismuth oxide and iron oxide plate with HARPOON again. They used the mesh that has an obvious coating of fluorescent paint and were able to see the orange color after purging with N2 for 40 min. They took pictures every 30 seconds for the first 10 min and every min for the next 10 min. The UV flashlight was flickering throughout the experiment (it probably needs new batteries!), so they’ll see how that affected the results when they process the data.
Week of 12/5-12/11
Almost everyone submitted an update this week! Sorry for the long post, but there is lots of news to share.
Alverno has continued with their efforts to calibrate their SEAL kit. Unfortunately they have been getting different readings for their plates with subsequent testings. They have been using plates from last year and the summer for these tests, which may be too old so they are making new iron oxide plates to test for reproducibility. Crescenta Valley made NiCl2 and ZnCl2 solutions to test next week. This week they tested different molarities of iron (III). The results weren’t great but a checkerboard pattern appeared showing the kit is working. Poly tried HARPOON again this week with the old, scratched mesh. They used an old SEAL plate with the wire cut off, but didn’t get any results after 20 min. Franklin’s Las Chemists got good results with the kit, so your two groups should talk!
Oakwood has some important updates on the UV-ozone cleaner. It was tested extensively by the summer students who discovered it was best to have a tiny layer of water on the template so it could stick to the glass and that only 5-10 minutes in the ozone cleaner was required to clean the glass and make it hydrophilic. Oakwood has since tried making the template better. The vinyl template was thin, seemed to degrade with exposure to the UV light, and irreversibly stretched. They tested some new materials and discovered the best material is a thin silicone which is thin enough to not cast a shadow, flexible enough to stay on the surface with a thin coating of water to help it stick, and seems to be robust. Oakwood has a laser cutter that was used to create holes in the perfect locations for the template. They just need to cut the template to the correct size for the smaller glass size. They might be able to create a bunch of these templates for other schools!
Beckman Group 1 tested the UV treated plate with Fe(NO3)3 in the sulfite solution bath. Part of the plate (one corner with three spots) were in the light blue region, which marked significantly higher results than the rest of the plate. They also had two other UV treated plates with Ni(NO3)2 and Cu(NO3)3, which they are planning to test next week. They also tested the first iron plate that they made in the first week for practice; results were deep blue all across. Group 2 made two different solutions: 0.3M Al(NO3)3 and Ni(NO3)2. They also diluted 0.1M Fe(NO3)3 to create 0.03M Fe(NO3)3. They plan on spotting a FTO plate with these three solutions in different ratios next week. They decided to experiment with aluminum and nickel, as research indicated that aluminum would allow for better adherence and nickel would allow for smaller particle size for more uniformity. Group 3 had made a 0.2M NaOH and 0.2M sodium sulfite solution last week. When testing their plates with spots with different ratios of 0.1 M Iron (III) nitrate, 0.1 M Copper (II) nitrate, and 0.1 M Nickel (II) nitrate, they used a 1:1 ratio of NaOH and NaSO3 for the bath used in the SEAL kit. They plan on analyzing their numerical results next week. Group 4 planned to run their UV treated 0.03M Fe(NO3)3 plate. However, their epoxy did not dry in time and they were unable to run the plates in this meeting. They also spotted their non-UV treated 0.03M Fe(NO3)3 plate and plan on running this plate next week.
Concordia started reading Astrid’s Chemical Reviews article and left off at the topic of fossil fuels. Then they started working with their CON8 plate (which is??). They added sodium sulfite to 100 mL of water in a Petri dish, and set up the SEAL kit, but they were a bit unfamiliar with using the kit and ran through the program twice but still ended up with an error. Feel free to ask for some help here!
PCC made a few more plates. Page spotted pure 0.05M bismuth(III) nitrate and pure saturated tungsten sulfide (exact concentration not yet known); 0.01M bismuth(III) nitrate. 1:1, 1:10, 10:1 ratios of 0.05M bismuth(III) nitrate and tungsten sulfide; 1:1 ratios of tungsten sulfide and iron(III) nitrate; 0.01M iron(III) nitrate. Ben spotted pure 0.05M bismuth(III) nitrate and pure 0.05M ammonium molybdenate; bismuth(III) nitrate and ammonium molybdenate, (NH4)6Mo7O24, in 1:1, 10:1, 50:1, 100:1, 1:10, 1:50, 1:100 ratios. When the bismuth(III) nitrate and ammonium molybdenate were mixed, a white precipitate fell out of solution. Although it is likely that this will crust off the plate once it is baked in the kiln, the solution w/ precipitate was still suspended and spotted onto the plate. The exact chemical precipitated has not yet been determined.
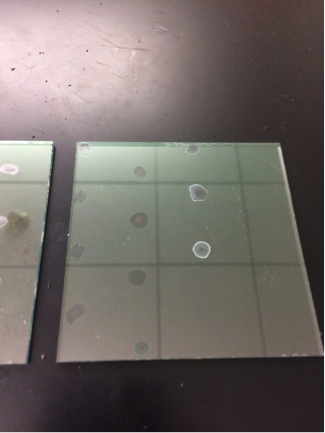
San Marino’s Red Team worked exclusively on making two more new plates to test later: one primarily focused on Manganese (II) Chloride with Nickel (II) Nitrate, Copper (II) Sulfate, and Cobalt (II) Chloride in 0.1 M concentration and 10 microliters spot size; the other is Nickel (II) Nitrate with Copper (II) Sulfate in varying ratios of Ni:Cu in 10 microliter spot size. The first plate is to re-test whether or not manganese was to blame for flaking or if it was another material, while the 2nd plate is to test the varying ratios of nickel to copper without the danger of manganese flaking and messing with data.
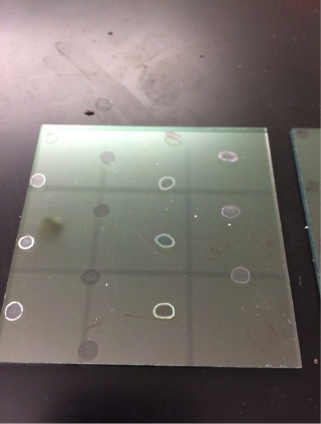
Here is plate 1 after drop-casting: (the copper and manganese turned white after cooling from bright orange. It is the third row.)
Here is plate 2 after drop-casting: As the amount of copper increased, the drops grew increasingly more white colored.
Lastly, Mayfield’s RAM team remade plates 6 (CuW, CoW, ZnW) and 7 . They also made plate #8 (CuWFe, CoWFe, NiWFe) all 10:6:4, which is similar to plate 6 just with the addition of iron. While heating, plate 6 cracked due to overheating. However, team KEN has successfully tested a cracked plate, so RAM will test the cracked plate next time. Team SEA is almost done with making plates with varying ratios of colored to non-colored metal salts and will be testing many of the plates in the new year. Team KEN had been testing some plates with a negative bias voltage (-0.1) and got results in the yellow range as the background dark current, with inverted results going down. They want to figure out chemistry for negative applied voltage to be able to test and interpret results. They spent rest of meeting discussing the SEAL experiment and chemistry. If you have any experience with applying a negative bias voltage, please share in the comments!
Thanks for reading all the updates and thanks to all our teams for posting and doing such great work. I’ll be sending invites to the CCI Solar Annual Meeting on Jan 28 at the beginning of the new year.
Week of 11/28-12/4
We’re still not consistently getting updates from everyone, so please try to remember to post in your google drive weekly (even if just to inform me you didn’t meet this week). Thanks to everyone who has been commenting on the blog- there are some great conversations happening!
First up, Beckman HS took a tip from their mentor, Astrid, and prepared a 0.2 M NaOH and 0.2 M sodium sulfite solution as she said sulfite led to favorable reactions and also helped with evidence comparison. Next week, they plan to use their new solution on a run with the SEAL kit. They are working on comparing plates that have been treated with UV and those that haven’t to see how the UV-ozone cleaning treatment affects the results. They plan to test both treated and untreated 0.03M iron nitrate plates next time. Another group also made 0.1M iron nitrate plates, both treated and untreated, though they had already established that lower molarities would lead to more homogenous spots, and 0.1 M solution is much too concentrated. Some materials that their teacher, Mr. Beilin, suggested further research in were aluminum and nickel, which are said to have smaller particle size and greater adhesion, respectively.This week they tested a 0.05M iron nitrate plate (what were the results??) and planned to test a couple others (0.04M copper nitrate and 0.04M nickel nitrate) but the epoxy wasn’t dry in time.
Crescenta Valley made a plate with .01 M WO3, but the solution tended to dissociate after sitting still for a few minutes, so they don’t know how well the spots are going to dry. They left it to air dry and will check back next time. PCC worked on spotting a few plates this week. Summer spotted nickel(II) nitrate and niobium chloride in a 10:1 ratio; bismuth(III) nitrate and niobium chloride in ratios of 3:1 and 10:1; bismuth(III) nitrate and niobium chloride in a 5:1 ratio; pure nickel nitrate and niobium chloride. Left to air dry. Haya spotted pure MoSe2; Bi(NO3)3 and MoSe2 in ratios of 1:1, 50:1, and 75:1; Bi(NO3)3 and NbCl5 in ratios of 1:1, 50:1, and 75:1. Left to air dry. Nick spotted half of the plate with pure Cu(No)2 and the other half of the plate with FeCl3 to practice spotting plates for the first time.
While waiting on news about the HARPOON mesh, Poly decided to replicate work from last year on bismuth samples. They tried pre-charging a bismuth nitrate plate, by applying 0.5 V to the plate for a minute, before each scan with the SEAL kit. However, this failed to get very reactive results. They hypothesized that perhaps the tilt of the plate in the container with sodium hydroxide solution prevented some spots from being exposed to the LEDs, so they switched to a larger, flatter container, which helped slightly. With pre-charging, they got roughly the same results from each scan of the plate, showing that this method is viable for improving reactivity, and reversing the effects of degradation in the basic solution. Here are some of the results:
They think there might be a ceiling to this method though, because, after they had fixed the tilt in the plate, they got similar results to those from the previous scans. While the pre-charging method is supported by the results, there’s a limit to it, and the lack of reactivity in the final scan may have simply been the result of too much degradation, as the plate, over the course of several scans, had been in the base solution for nearly 40 minutes. They spotted more bismuth and bismuth-iron plates for testing next week.
San Marino’s Wand Team noticed some changes to the spots after firing their plate. Originally, the spots were black, but the black spots transformed into a colorful array. The cobalt spot turned a pastel pink, the newly green spot was copper, the light yellow spot was iron, and the manganese spot became light brown. When they placed the plate into the potassium hydroxide solution, the manganese and iron began to corrode. They realized this as it was hard to stabilize the dark current, and they visually saw the two manganese and iron spots corrode. They also had a couple of technical difficulties the voltmeter, switching between two voltmeters a few times until they were able to get a relatively stable dark current.
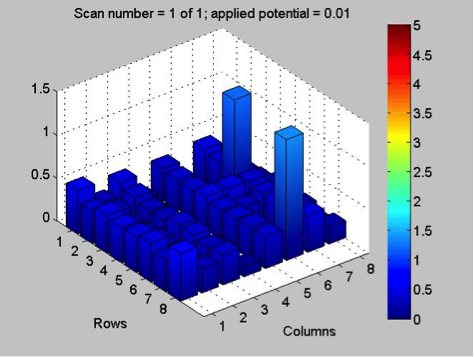
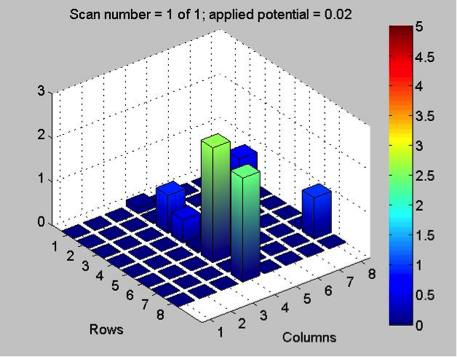
The Red team tested their plate with a combination with 0.1 Molar of manganese, nickel, and copper. The first scan (shown on the far left) showed that only two spots showed a significant result. These spots, they believe contain copper. The second scan (the middle) was interesting because they got a color other than blue! On these spots, they believe had some amount of manganese involved but are not entirely sure. They also think that this plate might not be conclusive of anything because there was a lot of flaking. The third scan (shown on the far right) shows that one spot was higher than the others. That spot was the pure copper spot of 10 microliters of 0.1 M copper.
Mayfield’s SEA team continued their work making plates with varying ratios of colored vs non-colored metal salts, specifically Co, Fe & Ni with Zn, W and Bi. The RAM team was having issues with the dark current on the latest several plates they made when attempting to test them. After some trouble shooting it looks like they were spotted on the wrong side, so they will be remaking them. Finally, team KEN tested their FeZn and FeNi plates to only mediocre results. However their CoZn plate had relatively high current throughout. The two spots with highest current occurred where there was no material deposited, which is a bit odd, but the overall high current warrants further testing of the CoZn materials.
Week of 11/21-11/27
With the holiday week, only a few teams had meetings this week. Poly is waiting for the HARPOON mesh to be tested by Franklin to confirm that it works, so in the meantime they spotted a couple of bismuth-iron plates. They plan on testing their plates with both SEAL and HARPOON.
Only Franklin’s Las Chemists team has been updating so far. Their group got to practice setting up the HARPOON kit, but unfortunately they spotted their materials on the wrong side of the FTO plate. They remade their plate and placed in the kiln to try next time.
San Marino’s Red Team worked on two things: reviewed an article regarding Photosystem II, and prepared another drop-cast plate since the first plate flaked. Samuel, a member of Red Team, found an article that removed the manganese atoms in the chlorophyll molecules of photosystem II, increasing the rate of hydrogen evolution. In water splitting, both the oxygen and the hydrogens are evolved, with both competing for space in the chlorophyll molecule. By removing the manganese, there was more space available for the hydrogen evolving process. With this new information, they plan to find out how to make the hydrogen evolving more efficient or increase the amount of hydrogen evolving occurring in a molecule of chlorophyll. With the plates, they decided to change the number of microliters deposited on the plate, the molarity of the samples, and the arrangement of the samples. In this new design, we placed columns of copper, copper with nickel, nickel, nickel with manganese, manganese, and manganese with copper. Each sample is from column to column, skipping one space after a sample to prevent them from blending together. 23 and 25 are 1.00 M because they want to see whether or not the molarity or the amount of the sample causes flaking. The corner box indicates which dots are left empty for the epoxy.
Crescenta Valley has been growing their membership base and are now up to twenty-some members, split into three teams each pursuing separate research goals. So far, they have been teaching new members, so they have made few new plates, but initial test results on 0.05 M iron(III) nitrate are deep blue (teams with successful iron results- like PCC- offer some advice to CVHS). They are planning on doing further testing with cobalt chloride and bismuth vanadate once the new members are settled. They are also planning on implementing the 3-D printed holder for the SEAL kit developed by Fairmont State once they get it printed.
Lastly, the Alverno team is just getting ready to start new experiments. They have spent the last few weeks re-testing plates used by the summer team and comparing the results. The results seem to match, so they are ready to move forward using their kit to test new materials. From running the plates, they also conclude that using bismuth and possibly some other metal give good photoactivity. They plan to focus on the use of metal combinations of bismuth with another semiconductor metal salt. Alverno also has a proposition to the rest of the teams- respond in the comments if you are interested:
“From our past years with our kit, we usually got low results with our kit for plates, even with the Iron standards. We have also talked with other teams before, and some mentioned they had had similar problems. This year, to follow up on the calibration of our kit, we are hoping to introduce another aspect to SEAL. We noticed that depending on the labs, kilning, materials, or maybe the kit, results could vary for different metals; Is any team interested in participating in a kind of collaborative study between the teams? We would be willing to run any plates that any teams have made with their various combinations of metals, and send our results (and return the plates) to see if they match up–just like what we did with the Summer SEAL plates. This could help to test any possible lab factors and also whether or not our kits are accurate. After we have begun creating our plates for our SEAL project, we would also like to send ours to other team’s labs to see if their kit also got similar results. If any teams would like to participate in such a study, please let us know.”