Almost everyone submitted an update this week! Sorry for the long post, but there is lots of news to share.
Alverno has continued with their efforts to calibrate their SEAL kit. Unfortunately they have been getting different readings for their plates with subsequent testings. They have been using plates from last year and the summer for these tests, which may be too old so they are making new iron oxide plates to test for reproducibility. Crescenta Valley made NiCl2 and ZnCl2 solutions to test next week. This week they tested different molarities of iron (III). The results weren’t great but a checkerboard pattern appeared showing the kit is working. Poly tried HARPOON again this week with the old, scratched mesh. They used an old SEAL plate with the wire cut off, but didn’t get any results after 20 min. Franklin’s Las Chemists got good results with the kit, so your two groups should talk!
Oakwood has some important updates on the UV-ozone cleaner. It was tested extensively by the summer students who discovered it was best to have a tiny layer of water on the template so it could stick to the glass and that only 5-10 minutes in the ozone cleaner was required to clean the glass and make it hydrophilic. Oakwood has since tried making the template better. The vinyl template was thin, seemed to degrade with exposure to the UV light, and irreversibly stretched. They tested some new materials and discovered the best material is a thin silicone which is thin enough to not cast a shadow, flexible enough to stay on the surface with a thin coating of water to help it stick, and seems to be robust. Oakwood has a laser cutter that was used to create holes in the perfect locations for the template. They just need to cut the template to the correct size for the smaller glass size. They might be able to create a bunch of these templates for other schools!
Beckman Group 1 tested the UV treated plate with Fe(NO3)3 in the sulfite solution bath. Part of the plate (one corner with three spots) were in the light blue region, which marked significantly higher results than the rest of the plate. They also had two other UV treated plates with Ni(NO3)2 and Cu(NO3)3, which they are planning to test next week. They also tested the first iron plate that they made in the first week for practice; results were deep blue all across. Group 2 made two different solutions: 0.3M Al(NO3)3 and Ni(NO3)2. They also diluted 0.1M Fe(NO3)3 to create 0.03M Fe(NO3)3. They plan on spotting a FTO plate with these three solutions in different ratios next week. They decided to experiment with aluminum and nickel, as research indicated that aluminum would allow for better adherence and nickel would allow for smaller particle size for more uniformity. Group 3 had made a 0.2M NaOH and 0.2M sodium sulfite solution last week. When testing their plates with spots with different ratios of 0.1 M Iron (III) nitrate, 0.1 M Copper (II) nitrate, and 0.1 M Nickel (II) nitrate, they used a 1:1 ratio of NaOH and NaSO3 for the bath used in the SEAL kit. They plan on analyzing their numerical results next week. Group 4 planned to run their UV treated 0.03M Fe(NO3)3 plate. However, their epoxy did not dry in time and they were unable to run the plates in this meeting. They also spotted their non-UV treated 0.03M Fe(NO3)3 plate and plan on running this plate next week.
Concordia started reading Astrid’s Chemical Reviews article and left off at the topic of fossil fuels. Then they started working with their CON8 plate (which is??). They added sodium sulfite to 100 mL of water in a Petri dish, and set up the SEAL kit, but they were a bit unfamiliar with using the kit and ran through the program twice but still ended up with an error. Feel free to ask for some help here!
PCC made a few more plates. Page spotted pure 0.05M bismuth(III) nitrate and pure saturated tungsten sulfide (exact concentration not yet known); 0.01M bismuth(III) nitrate. 1:1, 1:10, 10:1 ratios of 0.05M bismuth(III) nitrate and tungsten sulfide; 1:1 ratios of tungsten sulfide and iron(III) nitrate; 0.01M iron(III) nitrate. Ben spotted pure 0.05M bismuth(III) nitrate and pure 0.05M ammonium molybdenate; bismuth(III) nitrate and ammonium molybdenate, (NH4)6Mo7O24, in 1:1, 10:1, 50:1, 100:1, 1:10, 1:50, 1:100 ratios. When the bismuth(III) nitrate and ammonium molybdenate were mixed, a white precipitate fell out of solution. Although it is likely that this will crust off the plate once it is baked in the kiln, the solution w/ precipitate was still suspended and spotted onto the plate. The exact chemical precipitated has not yet been determined.
San Marino’s Red Team worked exclusively on making two more new plates to test later: one primarily focused on Manganese (II) Chloride with Nickel (II) Nitrate, Copper (II) Sulfate, and Cobalt (II) Chloride in 0.1 M concentration and 10 microliters spot size; the other is Nickel (II) Nitrate with Copper (II) Sulfate in varying ratios of Ni:Cu in 10 microliter spot size. The first plate is to re-test whether or not manganese was to blame for flaking or if it was another material, while the 2nd plate is to test the varying ratios of nickel to copper without the danger of manganese flaking and messing with data.
Here is plate 1 after drop-casting: (the copper and manganese turned white after cooling from bright orange. It is the third row.)
Here is plate 2 after drop-casting: As the amount of copper increased, the drops grew increasingly more white colored.
Lastly, Mayfield’s RAM team remade plates 6 (CuW, CoW, ZnW) and 7 . They also made plate #8 (CuWFe, CoWFe, NiWFe) all 10:6:4, which is similar to plate 6 just with the addition of iron. While heating, plate 6 cracked due to overheating. However, team KEN has successfully tested a cracked plate, so RAM will test the cracked plate next time. Team SEA is almost done with making plates with varying ratios of colored to non-colored metal salts and will be testing many of the plates in the new year. Team KEN had been testing some plates with a negative bias voltage (-0.1) and got results in the yellow range as the background dark current, with inverted results going down. They want to figure out chemistry for negative applied voltage to be able to test and interpret results. They spent rest of meeting discussing the SEAL experiment and chemistry. If you have any experience with applying a negative bias voltage, please share in the comments!
Thanks for reading all the updates and thanks to all our teams for posting and doing such great work. I’ll be sending invites to the CCI Solar Annual Meeting on Jan 28 at the beginning of the new year.

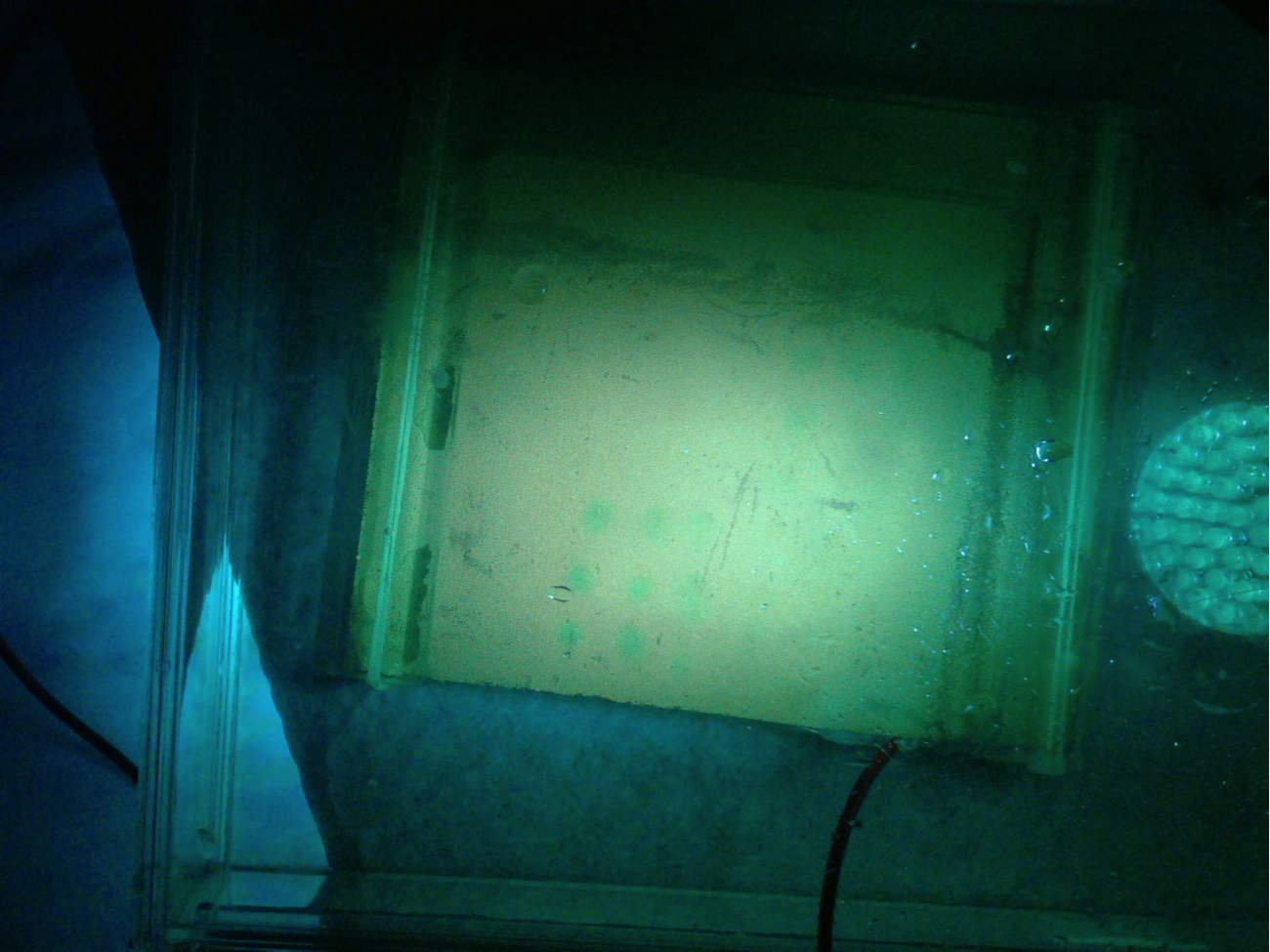
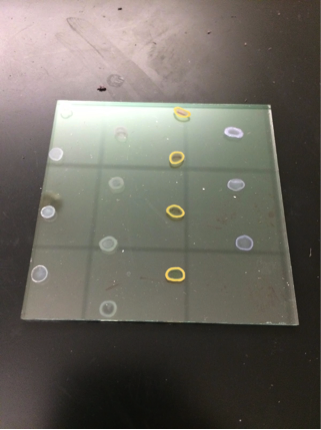
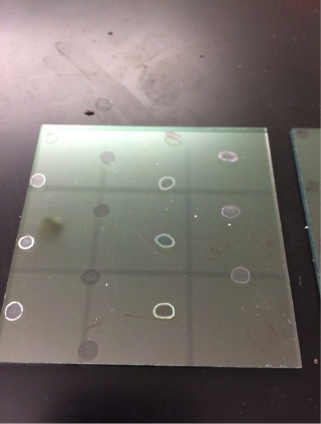
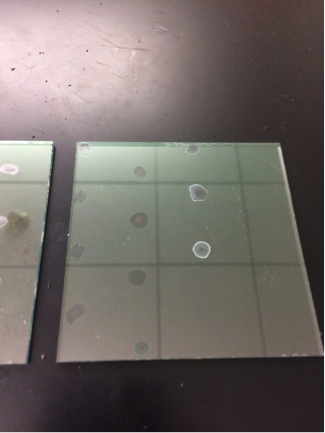
@Las Chemists – Our team is interested in learning more about how to use harpoon. We haven’t been getting very good results (actually none). The mesh we’re using is a faded yellow color. Since your team has had results, we would like to know about your methods of purging. Additionally, is your mesh a bright yellow color, or faded like ours?
@Agents of Poly Seal As a huge fan of Agents of Shield (which I am assuming you are referencing in your name), your name is so cool and Fitz Simmons will forever be a thing.