SoCal Infantry
Welcome to the Southern California SEAL infantry! SoCal SEAL students check here for important information regarding the SEAL program at Caltech
Important dates for 2019-2020 school year:
SEAL Kickoff: October 5, 2019 10am-12pm
SEAL Con XI: May 9, 2020 10am-2pm
2019-20 SEAL Roster
Every member of a SoCal SEAL team needs to sign-up on the roster. This ensures you will receive communications about upcoming events and helps us keep track of our membership. This also is how we make certificates for SEAL Con!
SEAL Permission Slip
All SoCal SEAL students need to fill out this permission slip and return it back to Kitty Cahalan either via email (kcahalan@caltech.edu) or in person through their mentor. Students under 18 must have the form signed by a parent or guardian.



Archives of SoCal SEAL Blog
Click on a blog post to view and add comments
Month of 12/19-1/15
Happy 2018 everyone! Now that we’re getting back into the swing of things after winter break, it’s time to share some SEAL updates. We have lots more this month ![]()
Alverno has posted their first update! They are divided into four groups this year: CO2 Reduction, Classic SEAL, Acid SEAL and Deconstructed Oxide Laboratory Printer Happily Inking Nonfoods (DOLPHIN) SEAL.The goal of the CO2 Reduction group is to utilize the SEAL kit in a setup that allows them to perform CO2 reduction by bubbling CO2 from subliming dry ice into the electrolyte. They will be looking at titanium dioxide, zinc, nickel for their efficacy at photoactive reduction of CO2.
The Classic group created some protocols such as using the kiln or spotting plates that will be uploaded to protocols.io soon. Last year, Alverno struggled with consistency but this year have had much more success replicating results for Fe and Ni plates. Next up is testing Manganese and Calcium salts as found in Photosystem II. The plates made in Classic SEAL can then be used by other groups, such as comparing the results in sodium hydroxide solution with the results Acid SEAL might get in hydrochloric acid.
The DOLPHIN group is designing a printer this year that can print out spots rather than pipetting them— this could help with making sure the spots are aligned with the template and are consistent. There’s some programming involved and it’s unclear which metal solutions will be compatible with the printer, but they are excited to see what happens!
Beckman’s Group 1 made ratios of 1:1, 1:2, 1:3 and 1:4 of 0.03M Cr(NO3)3, 0.03M Cd(NO3)2. They UV treated plate BHS-1-50 for five minutes and then spotted the plate with different ratios, including 0.03M Fe(NO3)3 standard spot in the top right corner.
Group 2 tested a plate they made previously which included a chromium nitrate, aluminum nitrate, and cadmium nitrate solution. They also spotted a chromium nitrate FTO plate and plan to test it next week.
Group 3 spotted BHS-1-60 with ratios of 2:1, 1:2, and 1:1 of 0.03M Fe(NO3)3 and 0.03M Al(NO3)3, with the iron standard in the top right corner. Iron nitrate is a significant and well-known photoanode while aluminum nitrate may further enhance the effective OER (oxygen evolution reaction) properties. Therefore, the main goal for the next session is to research and test another effective OER catalyst according to the results obtained with this plate.
Group 4 is testing the effects of varying lengths of UV treatments on the FTO plate. They first exposed an FTO plate to the UV treatment in the germicidal cabinet for thirty minutes and spotted with Fe(NO3)3, let it dry, then baked in the kiln. They repeated this process with a new plate for 15 min, 10 min and 5 min. They will compare the results of these plates to see if longer UV exposure time makes a difference.
Canyon Crest‘s first update as well. They had some issues with solutions evaporating over break, but were able to remake them and spot 5(!) plates shown below:
This meeting we were also able to use some odder solutions including using potassium dichromate (K2Cr2O7) which they had to stabilize with Nitric Acid. Mixing this with a 0.1M solution of CrCl3 we created a 0.1M chromium dichromate solution, Cr2(CrO7)3 (aq). They were surprised at its solubility, and will keep it for next meeting. They also speculated about isolating titanium in solution – converting between the titanium dioxide available in the stockroom and usable titanium tetrachloride by using nitric acid. However, they need to find a way to add chloride ions to the equation…
Also here is a link they found to a recently published Columbia University study on a new technology for seawater electrolysis which yields Hydrogen fuel as a product!
San Marino‘s Blank Team focused on the copper nitrate “incident.” They explain “We know something is wrong because nearly every spot on the plate began the test with a “bar” (see pictures of test runs) of the same level (green or blue-green). We also know that the problem has something to do with the machine/kit. The spots that were “outliers” (not having the same level as all the others) were made from a solution of only a copper salt.” Thoughts for them?
Green Team re-spotted their 10-31 plate, with Fe3+, Fe2+, Cu2+, Co2+. First scan produced nothing of significance. It was mostly dark blue. The lone outstanding spot was 50:50 Fe3+ and Zn2+. Spots began to crack and flake in the plate. The dark current was around 0.3V. (Sound familiar MSS KEY team? You should compare notes!)
Mayfield‘s Team KEY made plates to test additional metals with the successful FeZn material. 9:1:1 FeZnMg; FeZnNi; FeZnCu; FeZnAl; FeZnCo. Team JES had to remake their plates 3, 4 & 5 which to refresh your memory are a series of plates with Fe(NO3)3 and Ni(NO3)2 layered with various applications of a tesla coil. Plate 3 is Fe & Ni layered with 3 min of Tesla coil between the layers, plate 4 is treated with Tesla coil before the Fe, and plate 5 is treated with the tesla coil after both layers. After testing this series they hope to move on to experimenting with absorption spectra and artificial photosynthesis.
Week of 12/12-12/18
Only update this week is from San Marino‘s Blank Team. They tested last week’s plate (see last week for template), though there was a considerable coffee ring effect and mild flaking, especially the center of the “pure” Zn spots.
Spots are all green (starting), about same level. The 4 spots for “pure” Cu is very low (dark blue). Another 75% Cu and 25% Zn spot is in the middle (lower than majority green bars, but higher than the “pure” Cu). “Pure” Cu spots around 0.1 and 0.2V, and the majority green bars are around 2V. Run again and starts out blue with varying peaks, but then goes to about the same level of blue (slight green). “Pure” Cu is still relatively low (blue). They expect something as photoactive Cu to have good results, so need to look at the delta difference and figure out what happened.
They spotted a new plate using mixtures of Ni(NO3)2 and ZnSO4 and had Cu(NO3)2 and FeNO3 controls. Each spot is 10 microliters, 0.1 M concentration of each solution. They will test this plate after winter break.
Week of 12/5-12/11
Updates have been thinning out from all of our teams… remember to post EVERY week! Even if it’s just to say you didn’t have a meeting.
Beckman has decided they will do a quick experiment to test the effects of using UV light (ie ozone cleaning) on their plates before spotting. They will be using iron nitrate as a control and varying the amount of time left in the germicidal cabinet with the UV light from 5 min up to 1 hour. This will allow them to leave plates in the cabinet as short amount of time as possible while still getting the benefits of reduced coffee rings. This info will also be useful to other schools who have such a cabinet and wish to do the same technique.
San Marino’s Blank team made a new plate this week once again using Cu(NO3)2 and ZnSO4. Control of Fe(NO3)3. Spot ratios (Cu:Zn) are 10 to 0; 7.5 to 2.5; 5 to 5; 2.5 to 7.5; and 0 to 10.
Green team has 2 updates. They remade the plate with Fe3+, Ce3+, and Co2+, from 11/14. Along with the ratios shown below, they also added 3 spots of Cu(NO3)2 and 2 spots of CuCl2. It was fired at < 450℃.
| Spot | Fe3+ | Ce3+ | Co2+ |
| 1 | 0 | 0 | 100 |
| 2 | 0 | 100 | 0 |
| 3 | 100 | 0 | 0 |
| 4 | 50 | 50 | 0 |
| 5 | 0 | 50 | 50 |
| 6 | 50 | 0 | 50 |
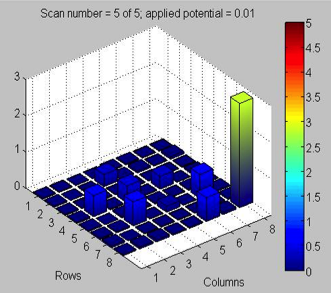
Upon testing, they found the two highest peaks corresponded to 100% Fe3. Usually, the Fe3+ control spot returns a light blue color, as it did at the beginning of the season. The fact that it was a darker color this time may indicate that a subpar material on one portion of the plate is impacting the performance of other spots. Changing the applied voltage from 0.05V to 0.1V depressed the peaks across the board.
They explain these results in the following manner: “As usual, the dark current was measured before each scan. It remained stable and below 0.5V, but we discussed that these checks don’t tell us everything. Corrosion and/or oxidation could be occurring on other parts of the plate. Essentially, there may be one or more “bad” materials on the plate that are acting as voltage leaks. Relative to the applied potential of our plate, they are “downhill”, meaning that any spike in current is hidden. Instead of jumping above the water splitting potential and releasing “downhill” to generate a current, it goes “through” the leak. Because the plate measures the difference in current when light is shined on a spot, a material that acts as a “leak” while in the dark can influence the spot being tested. One compelling fact is that even though our baking and testing proceeded without a hitch, we still got subpar results. In addition to our strange iron control spots, the Cu2+ spots on the side were not reaching the yellow/green range. The current leak concept is a possibility because it reveals why electrical activity may be masked. Next Monday, we will strive to eliminate the “bad” material combination, possibly by spotting multiple plates.”
Week of 11/28-12/4
Beckman: Made their first plates this week with different ratios of Cd(NO3)2, Fe(NO3)3, and Cr(NO3)3. They used a standard pattern to predetermine the locations of the iron standard spots to use consistently throughout the year. For the first plate, they used different ratios of the three solutions (1:1:1, 1:3:1, 3:1:1, and 1:1:3). For the second, they spotted the solutions separately, no mixing.
Poly: After analyzing results from last week they decided to use lower concentrations of the solutions, making it easier for the compounds to disperse. Planning to use a titanium oxide solution and an iron oxide solution first to make sure the setup is working. Last year they got good results from FeVO (only Fe worked best), so they may try to repeat this result and possibly TiVO to check the experimental set up.
San Marino: Blank team saw a lot of flaking on their Cu/Zn plate. No good results. The Fe control spot was also low & dark blue so something might be wrong with the plate. No new plates made this week, but plan to remake the Cu (II) nitrate plate from a few weeks ago that didn’t work due to copper wire exposed in the KOH solution.
Mayfield: Team JES got incredible results from their tests of plates 3 & 4 but this was likely in error as even areas with no spots had tall red bars. They will retest and compare to plate 5 next time. (Recap: Plate 3 is Fe & Ni layered with 3 min of Tesla coil between the layers, plate 4 was treated with Tesla coil before the Fe, and plate 5 was treated with the tesla coil after both layers).
Month of 10/22-11/27
It’s been a few weeks since the first blog post of the year. Now it’s time for a recap of the past month as more and more teams have added updates to their google drive docs. Excuse the extra long post. We’ll be back to weekly updates soon!
Beckman has split into 4 groups, each testing a different 0.03M solution- Cd(NO3)2, Cr(NO3)2, Al(NO3)2, & Fe(NO3)2. Crescenta Valley has 3 teams. Team 1 made a 0.04 M BiVO4 plate and is considering mixing other solutions with BiVO4 in future. Team 2 made 1:2 ratio CoCl2 and FeNO3 and plans to study coffee-ring effect and using tesla coil in future. Team 3 continued with 0.5M MnCl2 but added .1M CoCl2 to the plate this time. Plates will be tested in future weeks. PCC completed a round of 11 plates testing bismuth and various other metals. They made 0.05M solutions and tested mixtures of Bismuth + Tin (II), Bismuth + Tin (IV), Bismuth + Manganese, Bismuth + Aluminum, Bismuth + Zinc, Bismuth + Iron. Interestingly bismuth by itself had the best results shown below. They plan to increase the metal concentrations and try mixing more than two metals. They also mention trouble aligning every spot with the LED and will be working on a new template.
Poly decided to test potassium manganese and sodium manganese oxide because the band gap for both solutions was within 1.5 V, which is what we are looking for. Made 0.1 M MnO4 & 0.1 M KNO3 stock solutions and 11 dilutions of each which will be mixed in varying ratios from 100% Mn to 100% K. Did the same for Mn and Na. The plates were baked on a 300 degree hotplate for 1 min after spotting to prevent coffee rings. Testing showed low currents, but dark current readings may have been unreliable for a few of the tests.
Mayfield has 3 teams: FAM, JES & KEY. The first four plates FAM tested had combinations of Fe:Co, Fe:Cu, Fe:Ni, and Fe:Zn. The two plates that had the best results (not dark blue) were Fe:Ni and Fe:Zn, which has also been shown by a past Mayfield team. They also tried using a Tesla coil to decrease coffee-rings but had some trouble with spots spreading too wide. JES is studying light absorption by colored materials. They are basing their research off of summer 2015 research by Caitlyn Delgadillo and an iron and nickel layering technique. Based on their research of artificial photosynthesis, KEY wants to investigate magnesium cofactors, especially iron and nickel since they are earth abundant and tend to do well in photocatalytic materials. The ratios tested had low current and flaked into solution. They plan to look into stabilizing agents or other metal combinations found more commonly in chloroplasts.
Finally San Marino has lots of updates from their two teams over the past month.
Blank team: Spotted one plate using 5 varying concentrations of CuSO4 and ZnSO4 salts totaling 20 microliters per spot. After firing the Cu spots appeared white, but turned blue in solution and green upon removal. The current readings were all low, but the color changes were interesting so they remade the plate with CuCl2 instead of CuSO4 and only 10 microliter spots. They included spots of pure FeNO3, CuSO4, and ZnSO4 as controls. The wire was exposed and interfered with results so they will need to retest. They also remade this plate with CuSO4 instead of CuCl2 to directly compare to the other subteam’s results.
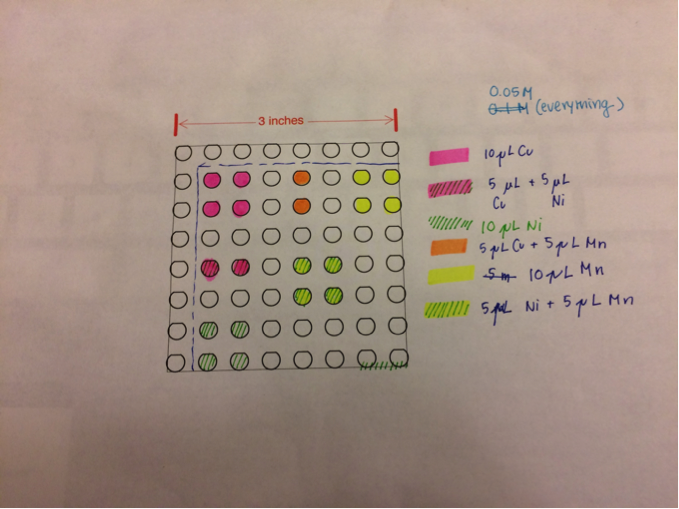
Green somethings: Tested a plate half (Fe2+, Fe3+, Cu2+) and half (Fe3+, Zn2+, Co2+). Only spots of 100% Cu2+ showed activity, everything else was dark blue. The spots were black and flaked during testing. Not sure if it was cuprous (I) or cupric (II) oxide, but Cu2O has a band gap of 1.2 eV, while CuO has 1.2eV. For the next plate, they chose to test Fe3+ (best performing iron cation), Ce3+ (from the Pourbaix diagram of electrical activity at pH 13), and Co2+ (also from the Pourbaix). Six ratios were made, alternating between 100:0 and 50:50 combos. All spots were 5 μL, and all solutions were 0.1M except for the Co2+, which was 0.05M. They also added a few spots of CuSO4 and CuCl2. The white cerium spots flaked during testing. Results were all dark blue. This could mean that solutions with Cu and Fe from 2 weeks ago could have been a fluke. A possible explanation is that amongst the group, there was a good catalyst and a bad light absorber, or everything was a medium catalyst. The difference in temperature at which the plates were fired could have also affected the performance of copper and iron on the plate. Two weeks ago, the plate was fired at 700°F but this plate was fired at 425°F.
Week of 10/16-10/22
First blog post of the school year! I know not everyone has started their research yet, but it’s important to record the week’s events in your Drive doc so we can record how you’re progressing. Even if it’s just the brainstorming stage of research, recording those thoughts and ideas is just as important as recording procedures and data. Sharing ideas with each other early on can lead to even better projects for the upcoming year.
So far we have updates from San Marino and Crescenta Valley. San Marino has 2 sub-teams. Team one did some great controls testing both Fe3+ ( iron nitrate) and Fe2+ (iron sulfate) to see what the difference between these compounds would be. Research of the Pourbaix Diagram for Fe2O3 from materialsproject.org showed that that this form of iron (III) oxide was electrically active at around 0.5 eV at pH 13, which was the pH in which we test our plates. As predicted the Fe3+ performed better (light teal) than the Fe2+ (dark blue). Next they added explored combinations of iron and copper, creating 6 ratios between Fe3+, Fe2+, and Cu2+, with the proportions being either 50:50 or 100:0. All solution were 0.10 M before mixing, and all the spots were pipetted and 5μL in size. Results coming soon!
The Blank Team at San Marino spotted, fired, and tested a control Fe(NO3)3 solution with varying concentrations of Fe(NO3)3. They wanted to test the effect of varied concentrations on the data as well as the durability of the spot. They tested four spots of 20 μL each (100%, 80%, 60%, 40%, and 20% Fe(NO3)3). After firing, a majority of the material fell off of the plate for the 60%, 30%, and 40% Fe(NO3)3 concentrations. The 100% Fe(NO3)3 and 80% Fe(NO3)3 concentrations still had material left on the plate, but they also lost some material as well. Results coming soon!
Crescenta Valley has split into 3 subgroups. Team 1: We made a 0.1 M BiVO4 solution. We want to continue our work from last year with BiVO4, and see how well BiVO4 works with Ni and Fe compared to Co. Team 2: Our goal this year is to experiment with different methods of alleviating the coffee ring effect. We have chosen three areas of focus: UV rays, a thin layer of silicone oil, and an ozone cleaner. This week we made a plate spotted with 0.1 M Fe(NO3)2 as a control plate. We plan to use the same solution in order to test the effects of the three methods listed previously. For the upcoming week, we plan to start buying parts for our ozone cleaner. Team 3: Our goal is to experiment with new materials and metals. After discussing with our mentors, we decided Mn with Ni might be a viable option based on a research paper we were looking at. More research will be needed to figure out how to make the Ni and Mn solution like in the paper. In the meantime, we made one plate with .1 M MnCl and .05 M NiCl, plated straight on top of each other. We will continue to experiment with new metals and see if we find anything.
Thanks for the updates CVHS and SMHS! Looking forward to hearing from everyone else soon. And remember to email you permission slips and sign up on the roster!!!!
Week of 4/10-4/16
BIG REMINDER: SEAL Con 8 is just under a month away on Sat May 13th!!! Everyone should be starting to prep their talks and posters and winding up experimental work. Since there will be less research happening and we want to leave final results for reveal at the conference, this will be the last “Week in Review” for the school year. Thanks everyone for your diligent updates and thoughtful comments and questions. Hopefully we’ll be able to make this blog work even better next year!
Very brief updates this week from San Marino and Poly. Throughout month of March, Poly continued to have issues with HARPOON data not showing up onto the software. Moving on they tried out a new website called the Materials Project, which is a huge database of compounds with information regarding band gap, stability, etc. After researching on the Materials Project database, they decided to testing a Yttrium Manganese compound by mixing manganese sulfate monohydrate and yttrium nitrate hexahydrate. They are currently making the material and will test it next week.
San Marino had some problems with their kiln getting too hot and damaging the FTO plates making them non-conductive. They will try and even lower setting next time to avoid this. They also have a new idea for testing plates without the SEAL kit. They would attach the leads of the voltmeter to the plate, attach and tape the black gel template to the bottom of the bowl and line up the plate with the spot on the bottom of the bowl. Then they would turn the setting on the voltmeter to current, such as 10 mA, and shine a flashlight and measure the current. No bias voltage! The other San Marino team had problems with the software and were unable to test but observed that their NaVO4, Fe(NO3)2, and CuSO4 materials turned into a yellow orange color. The spots with only NaVO4 washed off when they tried to test it. Next week they will try to test the plates again.
Thanks everyone and see you all at SEAL Con 8!! ![]()
Weeks of 3/20-4/9
Spring break is still affecting schedules so I’ve combined a few weeks of updates into one post. An official email will be going out soon, but just a reminder that SEAL Con is a month away! Now is the time to start wrapping up and finish those last few experiments so you can put together posters and talks for May 13.
Starting with an update from Alverno, they are continuing our work on the Acid project and CO2 Reduction Project. The finished plates for the Acid Project are Iron Nitrate, Zinc Nitrate, Nickel Nitrate and Copper Nitrate. The CO2 Reduction project has finished researching what is needed for it, and completed tissue plates of copper and nickel nitrate, copper and iron nitrate, copper and chromium chloride, copper and zinc nitrate, copper and bismuth vanadate, and copper and manganese sulfate. The ratios of the spots are 10:10, 15:5, 12:8, 5:15, 8:12, 2:18, 18:2, 13:7. The long-term goal is to reduce CO2 into Methanol. The group also finally solved some issues with a malfunctioning SEAL kit so they will be able to get results from all these plates in the coming weeks.
Mayfield’s Team SEA has been focusing efforts on a combination of iron and tungsten that produced good results in one combination on a previous plate. 3 future attempts at various ratios of iron and tungsten showed now significant results. They decided to try one more plate of Fe, W adding in some Co as well as a plate of just Co & Ni. They also are going to test plates of Ti & Fe and Ti, Fe & Cu. Team RAM’s attempts to lower the ratio of Bi and W in their Bi, V, Ni, W combinations didn’t prove to have any positive effect on the results. The next attempt will be to add more Ni and also try Cu instead of Ni with ratios of 8:1:1:8, 8:1:1:13, 8:1:1:16 Bi:V:W:Ni/Cu. Team PEAK is remaking their successful FeZn material which has been less and less reproducible overtime with a new Fe solution to see if that will improve activity again. They also started work on a voluntary(!) research paper to practice their scientific writing skills.
San Marino’s Wand Team made a new plate that consisted of sodium metavanadate base, cobalt chloride, cerium, and nickel solutions. The first solution was 20:10:20:0, second was 20:10:40:0, third was 20:0:10:20, fourth was 20:0:20:10, fifth was 20:10:0:20, and sixth was 20:15:10:10. Solutions one and two had precipitate. Solutions one, two, and six were orange; solutions three and four were yellow, and solution five was pink. Each spot on the plate had a total of 50 microliters of solution, and five spots of each solution. The plate was heated. Next week, they plan on testing this plate as well as the one made at the last meeting.
The Red Team drop-casted two plates: one with sodium vanadate, iron nitrate, copper sulfate and one with different ratios of just iron nitrate and sodium vanadate. The first plate has three sections with just sodium vanadate, iron nitrate, copper sulfate and three sections of (1) sodium vanadate and iron nitrate, (2) iron nitrate and copper sulfate, (3) sodium vanadate and copper sulfate. All of the solutions are 0.05 M and each space is 10 microliters total. The combinations have 5 microliters of each of the two included solutions. The second plate has groups of three dots, with each group of three having ratios of 10:0, 9:1, 8:2, 7:3, 6:4, and 5:5 microliters of sodium vanadate and iron nitrate. The last three ratios were in groups of two with 4:6, 3:7, 2:8, and 1:9 microliters of sodium vanadate and iron nitrate. All of the solutions are 0.05 M. Next week they plan to test these two plates and one made before spring break.
Finally Beckman HS has been busy. Last time, Group 1 decided that both Mn(NO3)2 and CuZn shared similar characteristics with effective photocatalysts and decided to make the solutions. As usual, the procedure consisted of the UV treatment prior to spotting, and they used the established 0.03M iron standard. They tested these two plates and made another plate to test next week. Group 2 made two plates last week: a plate of 0.03M Cu(NO3)2, 0.03M of Zn(NO3)2, and 0.03M Fe(NO3)3, in ratio of 1:1:3 respectively, and another plate of 0.03M of Cu(NO3)2, 0.03M Al(NO3)3 and, 0.03M Fe(NO3)3 in ratio of 1:1:3 respectively. Just like Group 1, these plates were UV treated before spotting, and were air-dryed and fired. They tested both plates and made an new plate with different ratios of 0.03M Copper (II) Nitrate, 0.03M Iron (III) Nitrate, and 0.03 Zinc Nitrate in a ratio of 1:3:1 respectively. When testing this plate they used a 1:1 ratio of 0.2M NaOH and 0.2M NaSO3 for the bath used in the SEAL kit. They plan on analyzing their numerical results next week.
Group 3 decided to make a new plate by diluting the 0.04M Co(NO3)2 to 0.02M. This was used to make a plate of 0.03M Fe(NO3)2 and 0.02M Co(NO3)2 with a ratio of 3:1. The reasoning for this is because when they tested BHS-3-51 last week, they surmised that a plate with more iron and less of the other metals would yield better results. And because of cobalt’s red color and physical properties, they decided to continue testing with cobalt again. The first plate tested was BHS-3-52, which was created by diluting 0.04M Cobalt Nitrate to 0.02M Cobalt Nitrate and and 0.04M Iron (II) Nitrate to 0.02M Iron (II) Nitrate and spotting the plate with different ratios. The second plate tested was BHS-3-53, which was created by diluting 0.04M Cobalt Nitrate to 0.02M Cobalt Nitrate and 0.04M Iron (II) Nitrate to 0.02M Iron (II) Nitrate, which was spotted on the plate with a 3:1 ratio respectively.
Last time, Group 4 began to prepare a 0.03 M Fe2(NO3) because the one that they did previously had an intense coffee ring effect. However, the spots were unfortunately not solid enough to test or even fire. They then spotted a multi-ratio plate with Iron (III) Nitrate and Copper (II) Nitrate in a ratio of 1 Fe: 1 Cu, 2 Fe: 1 Cu, 3 Fe: 1 Cu, 3 Fe: 2 Cu with a molarity of 0.05M. The plate was exposed to treated with UV light for five minutes. The plate will be air-dried and fired later; they plan on testing this plate next week. Aside from their respective labs, the 4 teams also were introduced to new lab techniques. They had a very brief introduction on how chromatography works and the need for almost needle-like, minuscule amounts of liquid, and proceeded to take turns making such micropipettes from ordinary capillary tubes.
Weeks of 3/6-3/19
With lots of schools on spring break, another 2 weeks have gone by. Same few teams have been posting, so thank you, but the rest of you please remember to update your google drive weekly. There have also been sadly very few comments and conversations on the blog so be sure to check the posts to see if you can answer or ask a question for a fellow team.
Poly worked up their HARPOON data after downloading the Image J software onto their laptops. Though they found no detectable oxygen evolution spots, they are determined not to give up and have reached out to the HARPOON experts at UW Oshkosh for some guidance. Good luck guys!
Mayfield’s Team SEA is zeroing in on a hit they got back with one of their colored vs non-colored tests. The combination was FeW so they have made two plates with varying ratios of the elements to test next time. Team RAM found results from BiVNiW 8:1:1:8, 8:1:1:13, 8:1:1:16 combinations to be ok, so they are making a new plate with the same metals, but less Bi and W (6:1:0.5:1, 6:1:0.5:5, 6:1:0.5:8). They also looked back at last year’s good results with BiVZn and decided to make a plate with BiVZnW to compare if adding W can help. Finally Team PEAK finished making plates 24 (CoZn 6:1 with layers) and 25 (FeZn 6:1 with layers). Results were not very good. Unfortunately all attempts at recreating a CoFeZn hit have gotten steadily worse overtime. They also made plate 26 (half CoZn 6:1, half FeZn 6:1) but the first layer was the Fe/Co and the second layer after drying on the hot plate was the Zn. This is to test whether different materials when applied in different layers make an impact on the plate’s success.
San Marino’s Red team were able to test only one plate that contains 10μL of combinations of Cu, Mn, and Ni because they spotted on the wrong side for one of the plates. They baked the plates at a higher temperature (around 650 C) which looks like it affected the FTO properties of the plate since the results showed no significant current. This week they made one plate using 3 different solutions: 1) 0.05M V; 2) 0.05M Fe + Cu; and 3) 0.05M V + Fe + Cu. They decided to keep the concentration at 0.05M because that is the “sweet spot” at which there is reduced or no flaking (as supported by previous plate tests/experiments). Each drop had a total of 10 microliters of solution. They added three drops of each solution at the three corners of the plate. Next week, they plan on 1) testing this week’s plate, and 2) creating an identical copy of this week’s plate, but with some surfactant (soap) to see if the lowered surface tension of the solutions will reduce the coffee ring effect of each drop.
Lastly Beckman HS decided to take some time to reflect and do a bit of research. Group 1 is looking for a new element that is a strong photoanode and looking for elements that fall within a certain bandgap. These elements need to be cost-effective and abundant. Last week they tested a Nickel (III) Nitrate plate that did not have good results with most of the graphs concentrated in the blue region. For this reason, they are looking for a better element rather than jumping in immediately. Some of the things they are now considering from their research are zinc-related and chromium-related compounds. They are going to make 0.04 M Manganese Nitrate(Mn(No3)2) and 0.04 M of CuZn solutions. They will spot two plates of each solution. Based on their research, they found that platinum iridium, titanium oxide, and ruthenium shared the characteristics of being white and having bandgaps between 1.2eV and 2.8eV. Group 1, then studied the band gaps of materials that were stored in the stockroom. Since Manganese Nitrate is both white and has a bandgap of 2.8 eV, they decided to try 0.04 M solution of it. They also found that CuZn has similar traits as platinum iridium.
Beckman’s Group 2 epoxied their 0.03M aluminum nitrate/copper (II) nitrate/iron (III) nitrate plate and will run it next week. They then researched other candidates for testing, looking into iron and its capabilities and other red oxides. They decided to experiment with old plates in order to clarify the unclear results that they got in the past. The areas that did not have spots still gave them results, which led them to suspect an error either from the plate or the box. Therefore, they planned to retest the plates (0.03M of Copper Nitrate (Cu(NO3)2), 0.03M Aluminum Nitrate (Al(NO3)3)and, 0.03M Iron Nitrate (Fe(No3)3) in ratio of 1:1:3 respectively). Also, they wanted to see how different the results will come out by using Zn(NO3)3 instead of Al(NO3)3 so they made another plate of 0.03 M Cu(NO3)2,0.03M of Zinc Nitrate (Zn(NO3)2), and 0.03M Fe(NO3)3, in ratio of 1:1:3 respectively. They plan to do UV treatment to both plates.
Group 3 is mixing different ratios of Cu (II) Nitrate and Iron (III) Nitrate that are both at 0.04M. They UV treated the plates before spotting and exposed them for five minutes. They tested the plate with coating method, which is spotting the plate with one compound and after they air dried it, then putting the second compound on. This did not work so they are making another plate by mixing two metal solutions in a fixed ratio. The resulting spots were very dark and they believed that this happened due to the high concentrations. Therefore they made a new plate with 0.02 M Co(NO3)-diluted from 0.04M CoNO3- and 0.02 M Fe(NO3)2-diluted from 0.04M Fe(NO3)2 with different ratios. They made their plate with a different Molarity because they found out that mixing method produced better results than that of coating method. However, they weren’t sure whether higher or lower concentration would give better results, which inspired them to test different molarities.
Group 4 ran their 0.03M Copper (II) Nitrate plate last week and the results were not that good. Most of the graph was in the blue zone indicating not strong results. This week, they researched other potential photoanodes and came across carbon nitride, which had not been heard of before. The American Chemistry Society website said it is a good photoanode that allotted high results, but it is not in the school inventory ![]()
Last week this group spotted 0.03MCo Nitrate 2 without the iron standard. The results were not satisfying because of intense coffee ring effect seen despite their usage of UV treatment. They believe that this may have been a procedural error during the drying stage, so they produced another plate with 0.03 M Co(NO3)2 so that they can make sure that the plate can be spotted with no procedural error, especially during the drying stage. The spots will be tested during the next meeting.
Week of 2/27-3/5
A couple more schools included updates this week, but many others haven’t posted since the Annual Meeting. We want to hear what you’ve been up to! Write up a quick recap and then begin weekly posting again. SEAL Con is just over 2 months away so now is the time to ask questions and get help so you can finish off your research project as best you can before May 13.
First up, Beckman HS Group 1 tested a Ni(NO3)3 0.04M plate, but it didn’t have very good results. They also prepared a plate with 0.04 M Fe(NO3)3 which had shown promising results last time on an identical plate. They are currently testing the third iteration of this plate. The first iteration had high results but the second seemed to have a leak in the epoxy, which may have affected the results, so they are following up with their results with a duplicate of the original plate. Group 2 is testing a plate with multiple elements (Cu(NO3)2, Fe(NO3)3, and Al(NO3)3 all with 0.03 M). They decided to do this after doing research last week and concluded that this combination might have promising results. In the long run, they plan to consider remaking and improving this plate, and perhaps altering the molarities and the ratios to see how that might affect their results. Group 3 is trying two techniques in plating. They used cobalt nitrate and iron nitrate and tried layering them on the plate (iron on top of cobalt), as well as mixing them and spotting them. They saw both ideas at the CCI conference and saw good results. For the future, they will check their results to see which method works better and use this as a basis for future trials and use this technique for other combinations if it does show positive results. Group 4 is testing a Cu(NO3)2 with 0.03M duplicate plate after getting some red results on the original. The new results were not outstanding, so they decided to try different metal nitrates but want to stay with the same ratio for future tests.
Next, Poly was finally able to run a HARPOON experiment! Their mentor, Josef, was able to fix the power supply box. They took pictures every 30 seconds for 15 min of a plate spotted with the HARPOON standard, Ni:Fe:CO 1:2:2. Next week they will do a data analysis of these photos in ImageJ as well as start on Raman Spectroscopy experiments.
San Marino’s Team Photosynthesis created two duplicate plates identical to the plates made previously following this configuration to ensure consistency:
They were unable to test previous plates since they lost one and the other was left in the kiln for too long, resulting in the warping of the FTO coat on the plate making it nonconductive. They used the metals (Cu, Mn, Ni) because they are found in a photosynthesizing protein. Manganese is suspected to be the material flaking, and expect to see brown flakes floating in the NaOH solution if so, as well as inconsistent measurements of current in specific areas. If there is flaking, they plan to ozone treat the plate and make an identical plate to test. If even after there is still flaking, they will ozone treat individual plates, with each metal having its own plate, isolating each metal from each other. Also, they are looking into the coffee-ring effect that can result from drop-casting, and will test the two identical plates next week and talk about what they found about the coffee-ring effect.
San Marino’s Team Wand, also could not test the plate they made last week because it had the same problem of the FTO melting. The temperature of the kiln was too high and the plate was left inside for too long, causing the FTO coating to melt and become warped, similar to cellophane. During this week’s meeting, they remade the plate that was ruined, which contained vanadate and varying ratios of nickel.
Concordia analyzed results from the CON8 plate that was zapped with the Tesla coil and concluded that it discolored the spots of bismuth nitrate and ammonium vanadate. The discolored spots that they used the Tesla coil on had very low results. Then, they brainstormed to figure out what to work on next. They recorded previously used metal oxide solutions and wrote down others which they would like to test. They didn’t officially decide what materials to test yet, but we prepared a blank plate for next time.
Finally Mayfield’s Team SEA tested the aluminum copper plate, but it did not produce quantifiable results. They researched a few other good conductive metals but for now will stick with the elements they already decided on, testing the iron chromium plate next week. Team RAM tested plate 14 that contains bismuth, vanadium, and nickel with the ratio of 8:1:8, 8:1:13, 8:1:16. The results we’re not bad and performed better than plates 11 and 12. They also spotted plate 15 containing bismuth, vanadium, nickel, and tungsten with the ratio of 8:1:1:8, 8:1:1:13, 8:1:1:16. Team PEAK tested plate 23 (split CoZn 6:1 and FeZn 6:1 with multiple layers), but only got dark blue results. They plan to test the same materials with layering again, but without annealing them in between adding the layers. The previous plate was annealed after adding the first layer, which caused large spreading of metal solution when the second layer was applied and may have impacted results in unexpected ways. They started making plates 24 (FeZn 6:1 with layers) and 25 (CoZn 6:1 with layers, which they will finish next meeting with the additional layers.