We’re still not consistently getting updates from everyone, so please try to remember to post in your google drive weekly (even if just to inform me you didn’t meet this week). Thanks to everyone who has been commenting on the blog- there are some great conversations happening!
First up, Beckman HS took a tip from their mentor, Astrid, and prepared a 0.2 M NaOH and 0.2 M sodium sulfite solution as she said sulfite led to favorable reactions and also helped with evidence comparison. Next week, they plan to use their new solution on a run with the SEAL kit. They are working on comparing plates that have been treated with UV and those that haven’t to see how the UV-ozone cleaning treatment affects the results. They plan to test both treated and untreated 0.03M iron nitrate plates next time. Another group also made 0.1M iron nitrate plates, both treated and untreated, though they had already established that lower molarities would lead to more homogenous spots, and 0.1 M solution is much too concentrated. Some materials that their teacher, Mr. Beilin, suggested further research in were aluminum and nickel, which are said to have smaller particle size and greater adhesion, respectively.This week they tested a 0.05M iron nitrate plate (what were the results??) and planned to test a couple others (0.04M copper nitrate and 0.04M nickel nitrate) but the epoxy wasn’t dry in time.
Crescenta Valley made a plate with .01 M WO3, but the solution tended to dissociate after sitting still for a few minutes, so they don’t know how well the spots are going to dry. They left it to air dry and will check back next time. PCC worked on spotting a few plates this week. Summer spotted nickel(II) nitrate and niobium chloride in a 10:1 ratio; bismuth(III) nitrate and niobium chloride in ratios of 3:1 and 10:1; bismuth(III) nitrate and niobium chloride in a 5:1 ratio; pure nickel nitrate and niobium chloride. Left to air dry. Haya spotted pure MoSe2; Bi(NO3)3 and MoSe2 in ratios of 1:1, 50:1, and 75:1; Bi(NO3)3 and NbCl5 in ratios of 1:1, 50:1, and 75:1. Left to air dry. Nick spotted half of the plate with pure Cu(No)2 and the other half of the plate with FeCl3 to practice spotting plates for the first time.
While waiting on news about the HARPOON mesh, Poly decided to replicate work from last year on bismuth samples. They tried pre-charging a bismuth nitrate plate, by applying 0.5 V to the plate for a minute, before each scan with the SEAL kit. However, this failed to get very reactive results. They hypothesized that perhaps the tilt of the plate in the container with sodium hydroxide solution prevented some spots from being exposed to the LEDs, so they switched to a larger, flatter container, which helped slightly. With pre-charging, they got roughly the same results from each scan of the plate, showing that this method is viable for improving reactivity, and reversing the effects of degradation in the basic solution. Here are some of the results:
They think there might be a ceiling to this method though, because, after they had fixed the tilt in the plate, they got similar results to those from the previous scans. While the pre-charging method is supported by the results, there’s a limit to it, and the lack of reactivity in the final scan may have simply been the result of too much degradation, as the plate, over the course of several scans, had been in the base solution for nearly 40 minutes. They spotted more bismuth and bismuth-iron plates for testing next week.
San Marino’s Wand Team noticed some changes to the spots after firing their plate. Originally, the spots were black, but the black spots transformed into a colorful array. The cobalt spot turned a pastel pink, the newly green spot was copper, the light yellow spot was iron, and the manganese spot became light brown. When they placed the plate into the potassium hydroxide solution, the manganese and iron began to corrode. They realized this as it was hard to stabilize the dark current, and they visually saw the two manganese and iron spots corrode. They also had a couple of technical difficulties the voltmeter, switching between two voltmeters a few times until they were able to get a relatively stable dark current.
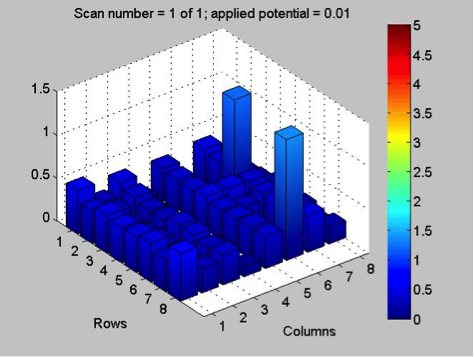
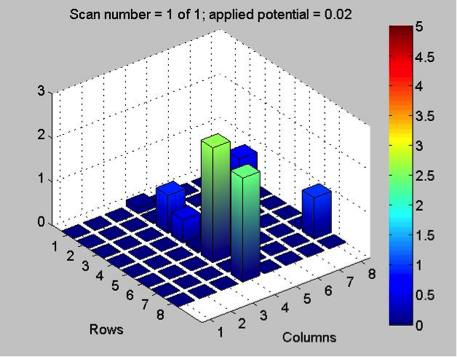
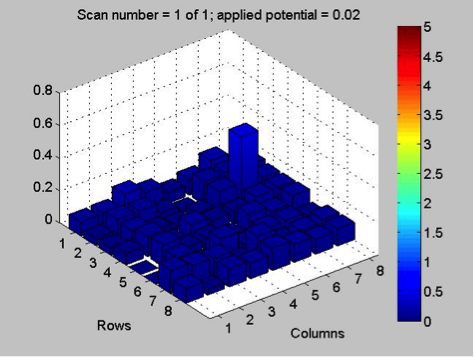
The Red team tested their plate with a combination with 0.1 Molar of manganese, nickel, and copper. The first scan (shown on the far left) showed that only two spots showed a significant result. These spots, they believe contain copper. The second scan (the middle) was interesting because they got a color other than blue! On these spots, they believe had some amount of manganese involved but are not entirely sure. They also think that this plate might not be conclusive of anything because there was a lot of flaking. The third scan (shown on the far right) shows that one spot was higher than the others. That spot was the pure copper spot of 10 microliters of 0.1 M copper.
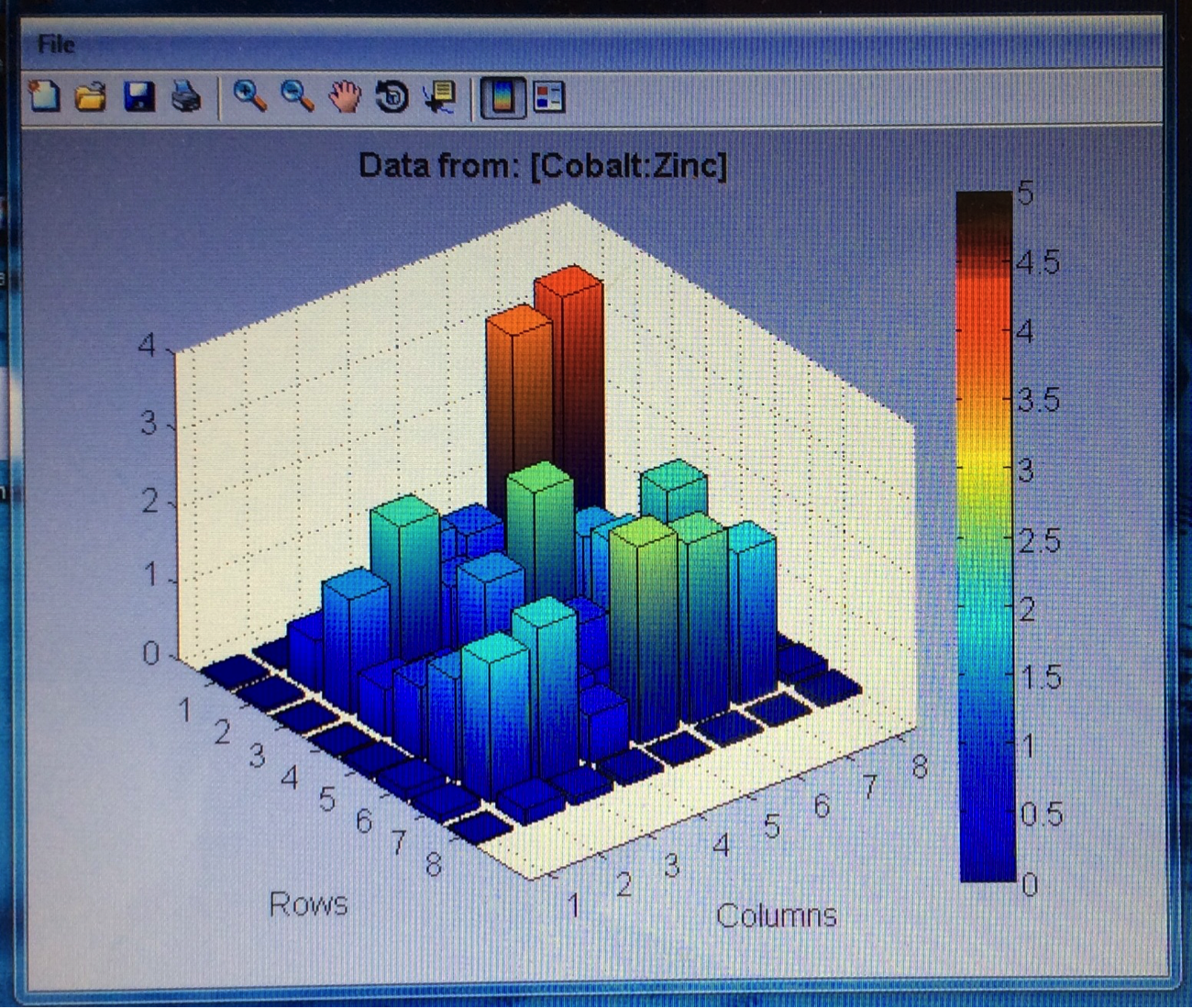
Mayfield’s SEA team continued their work making plates with varying ratios of colored vs non-colored metal salts, specifically Co, Fe & Ni with Zn, W and Bi. The RAM team was having issues with the dark current on the latest several plates they made when attempting to test them. After some trouble shooting it looks like they were spotted on the wrong side, so they will be remaking them. Finally, team KEN tested their FeZn and FeNi plates to only mediocre results. However their CoZn plate had relatively high current throughout. The two spots with highest current occurred where there was no material deposited, which is a bit odd, but the overall high current warrants further testing of the CoZn materials.

San Marino Wand Team – We would be interested to see a picture of your colorful spots, or even a video of the corrosion. Would you mind uploading a couple next time?
Crescenta Valley, do you have any ideas as to why your WO3 solution dissociated? We’ve been experiencing similar issues, particularly with our Fe solution; we believe this may be due to contamination, but we’re not entirely sure. Thanks!
San Marino Red Team: We see that you have been doing particularly well with copper and manganese. We are starting to work with copper too. Have you worked with other combinations including copper, manganese, with tungsten and iron? If so, how were your results? Thanks!
We have a question for everyone: In past conferences, we have put a lot of effort into the solving the coffee ring effect. Why do we put so much emphasis on this?
Hi webmaster, i’ve been reading your content for
some time and I really like coming back here. I can see that you probably don’t make money on your website.
I know one interesting method of earning money, I think you will like it.
Search google for: dracko’s tricks