SoCal Infantry
Welcome to the Southern California SEAL infantry! SoCal SEAL students check here for important information regarding the SEAL program at Caltech
Important dates for 2019-2020 school year:
SEAL Kickoff: October 5, 2019 10am-12pm
SEAL Con XI: May 9, 2020 10am-2pm
2019-20 SEAL Roster
Every member of a SoCal SEAL team needs to sign-up on the roster. This ensures you will receive communications about upcoming events and helps us keep track of our membership. This also is how we make certificates for SEAL Con!
SEAL Permission Slip
All SoCal SEAL students need to fill out this permission slip and return it back to Kitty Cahalan either via email (kcahalan@caltech.edu) or in person through their mentor. Students under 18 must have the form signed by a parent or guardian.



Archives of SoCal SEAL Blog
Click on a blog post to view and add comments
Week of 11/14-11/20
Posting to Google Drive and adding blog comments is now required for an invitation to the CCI Solar Annual Meeting in January. We’ve got a few more teams regularly updating, but hopefully we’ll have everyone regularly updating soon.
Beckman HS’s 4 groups are working with a UV ozone cleaner and testing various concentrations and combinations of iron, copper and nickel nitrates. Group 1 did the UV treatment once more on 0.04 M iron (III) nitrate because the last test had inaccurate spotting. Also, for the UV treatment, Group 1 used a new mask that only applied UV treatment on the spots, and checked to see the difference. Additionally, Group 1 made two new plates with 0.04 M copper nitrate and 0.04 M nickel nitrate to check for better results when applied with UV treatment during the next meeting. Group 2 tested and compared two plates of 0.1 M iron (III) nitrate with UV treatment and without UV treatment to see how well the UV treatment worked. Analysis is yet to be determined. Group 3 made a plate with spots with different ratios of 0.1 M iron (III) nitrate, 0.1 M copper (II) nitrate, and 0.1 M nickel (II) nitrate because of the possibility of better results when mixing multiple metal oxides, and the ratios were 1:1:1, 1:1:2, 1:2:1, and 2:1:1. During the next meeting, this plate will be tested and run with UV treatment, and further analysis will be shared. Group 4 did UV treatment on the 0.05 M iron (III) nitrate plate that was spotted and heated at the last meeting to test for results. Also, they spotted a 0.03 M Iron (III) nitrate plate but could not do UV treatment on the plate to see which molarity had better results due to a shortage of time.
PCC did some testing this week of 2 plates they made last month and had some incredible results! Benjamin tested iron nitrate, lead nitrate and bismuth nitrate in varying combinations. Page tested combinations of iron nitrate and bismuth nitrate, though she wasn’t at the meeting to confirm exact ratios. While the dark current of both plates wouldn’t settle to the 0.5 threshold (1.2, 0.9 for Benjamin’s plate and 1.8 for Page’s), the results show current far surpassing that background. Check out their results below:
The spots containing bismuth nitrate gave the highest measurements of photoactivity. The pure bismuth(II) nitrate spots measured close to 9, while iron(III) nitrate spots were around 3 and 4. The lead(II) nitrate spots gave very low values of approximately 1-2. Combining bismuth(III) nitrate with other solutions lowered the photoactivity of the bismuth nitrate.
Poly is still struggling with getting the HARPOON kit to be purged correctly and stay oxygen free for testing. Franklin HS- please send them some advice in the comments since you have the most experience with the kit! All they see if green despite having a purged solution from their mentor and a blanket of argon gas on top of the solution. Have you had any success with the mesh in the lab recently? Do we need new ones?
San Marino’s Red Team prepared the 2 plates (one electroplated and one drop casted) for testing with the SEAL kit. They applied 0.13 Volts and got about 0.015 microamps of dark current. Both plates only registered results in the blue region. However, much of the deposited metal oxides on the plates flaked off during testing. They speculate the reason for this is that they used a 1 molar concentration of some of the hydrated metal compounds instead of the normally used 0.1 molar concentration. Therefore, they plan on using 0.1 molar from now on when they do electroplating. For future meetings, they might also start focusing on the juice from juice research, where we will examine varying types of juices and their efficiency in creating a current flow.
Team KEN from Mayfield had poor luck last time repeating successful results from last year with FeZn. They theorize that the Fe solution last year was contaminated with nickel, and so might have skewed the results as FeNi have been known to work well. They created a new FeZn plate and FeNiZn and FeNi plates to determine them if the addition of zinc helped the results, and also if the solution was contaminated. Team RAM tested a CuW plate with moderate results (current in the light blue region). Unfortunately the second plate they tested with CuW, CoW, and ZnW had technical issues and there were no results. Try re-epoxying and test again girls! Lastly, team SEA tested a couple plates of their colored and non-colored metal salt combinations. While they didn’t have high current output, the 1:2 ratio of colored to uncolored metals produced slightly higher results than the plate with the 2:1 ratio of colored to uncolored. Team SEA has decided to work more with the more successful ratio, and also have decided to make new plates with a 1:3 or 1:4 ratio of colored to uncolored metals in the future.
Congratulations on all the hard work everyone! It’s exciting to start seeing results and the progress everyone is making!
Week of 11/7-11/13
Lots more updates this week! And we’ve had our first comments- Franklin and San Marino, check back at previous posts to answer some questions from fellow SEAL members.
Beckman HS is continuing work with their UV ozone cleaner. They are testing UV cleaning with iron (III) nitrate samples of various concentrations (0.1M, 0.04M, and 0.05M) as well as 0.04M copper (II) nitrate. They tested the 0.1M Fe(NO3)3 plate with the SEAL kit, but could not get the dark current to settle below 0.75 microamps.
The Oakwood team is also using their UV zone cleaner for a plate with iron salts, but with a lower concentration of 25mM. They used multiple different iron salts to test if the counterion or other additives affects the final iron oxide sample. After annealing, their iron plate had almost no coffee-ring effect and no flaking. Unfortunately they were unable to test the plate this time due to software issues.
The Poly team attempted to test one of their plates with the HARPOON kit for the first time. They had some trouble getting the solution purged and oxygen-free for testing. If you’ve done the HARPOON experiment before, please share some advice for the Poly team in the comments.
The San Marino Magic Wand team also had some software troubles this week, so they focused on making another plate to compare their dropcasted one from last time. The “magic wand” for electrodeposition was working so they were able to make a plate with 6 different samples with varying ratios of NaH2PO2, KH2PO4, and NiCl2. The samples with 1:4 ratios Ni:P gave a deep black color which seems promising, so three of the spots have that ratio. The plate will be annealed and ready for testing next time.
Finally, the three Mayfield subgroups have been working hard making lots of sample plates. SEA group is still working on testing their set of colored metal salts with non-colored salts. This week they made a plate comparing Bi, W, and Zn with Co, Fe and Ni. After some disappointing results from their BiVZn, BiZnCo, and BiZnCu samples, the RAM subgroup is modifying the samples to see if switching the metals will improve the activity. This week they made samples of CuW, CoW, and ZnW. Check out the beautiful colors of the samples below. The KEN team is building upon work they did last year with a successful FeZn material. Repeated testing of a new FeZn material yielded much lower results than last year, though spots closest to the epoxied wire did have significant current in the light blue region. Additions of cobalt to Fe and Zn and CoZn, tested with a varied ratio pattern, were unsuccessful.
Keep up the great work everyone. We’d love to hear from the rest of our teams too, so remember to update your Google Drive doc every week. Ask questions, comment, and get conversing with your fellow SEAL members. I know there’s lots you can learn from each other!
Week of 10/31-11/6
Very few updates this past week, but here are highlights from San Marino HS and catch-up updates from Team 3 (“Las Chemists”) from Franklin HS.
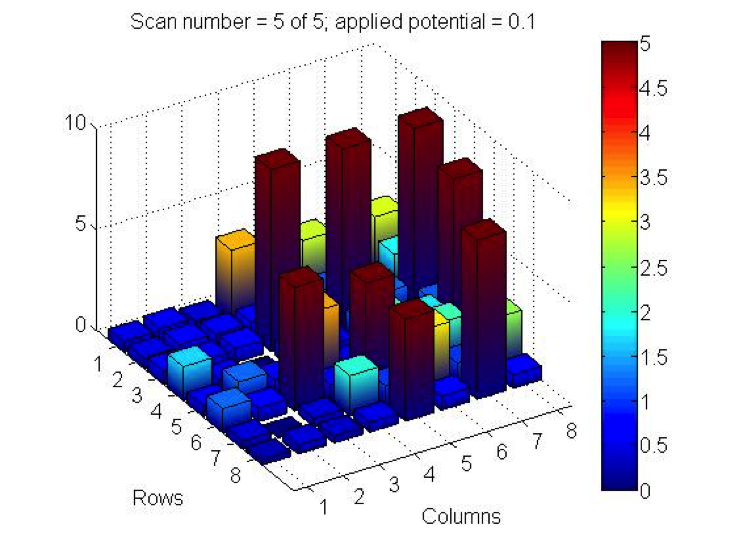
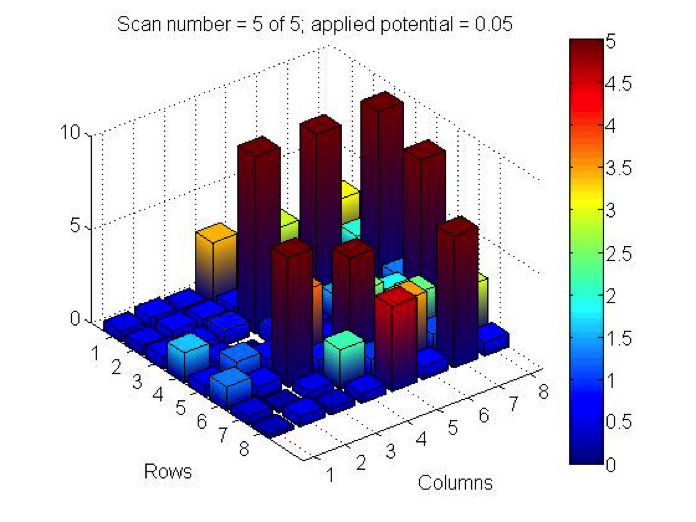
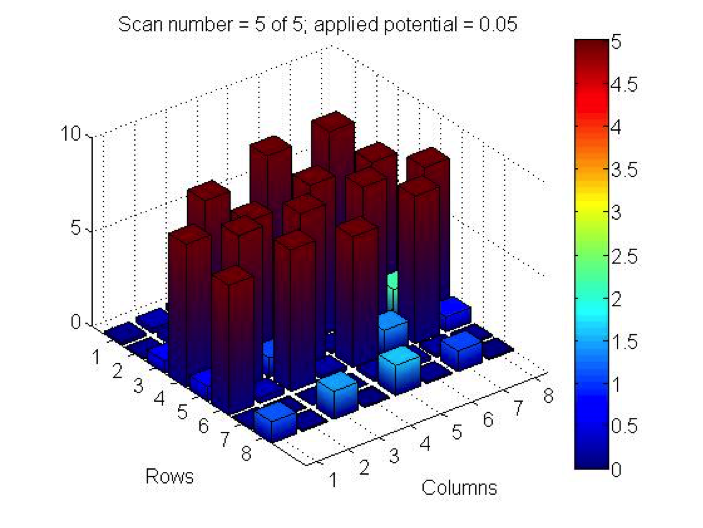
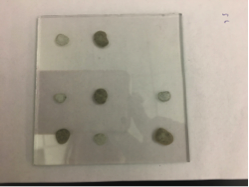
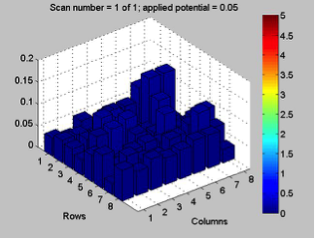
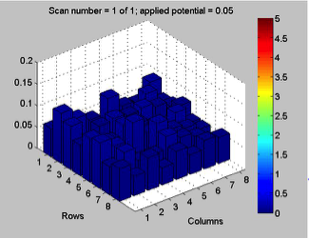
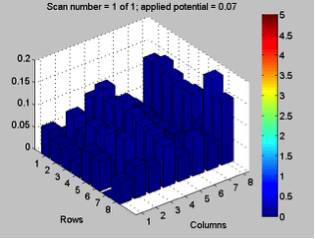
Red team from San Marino tested their plate from last time containing various combinations of 0.1 Molar of MnCl2, CuSO4, and Ni(NO3)2. None of the metal oxide spots resulted in higher current than the blank spots. They also planned to test blackberry juice as a dye in DSSCs with large and small surface areas of TiO2. Unfortunately they weren’t able to complete both tests before the sun set. Magic Wand Team tested their plate from last week with the dropcast Ni2P3 and NiP4 solutions. After putting the FTO plate in the kiln, the spots changed color from black to a green-brown (as shown below). They did three scans, the first two with an applied potential of 0.05, and the third with an applied potential of 0.07 (as shown below). None of the scans showed high current in any of the materials. Then, they made another FTO plate with the same solutions to test how different amounts of time on the hot plate could affect the solutions. Unfortunately the FTO plate shattered due to repeated changing temperatures of the hot plate and the lab table.
One of Franklin’s subgroups, Las Chemists, has documented their work so far this year on Google Drive. They decided on 4 metal salts to make solutions of: sodium molybdate dihydrate, cobalt chloride hexahydrate, nickel nitrate hexahydrate, and cobalt acetate tetrahydrate. After making their solutions, they also added 10% ethylene glycol to each solution (assuming for easier spotting? Let us know in the comments ladies!). The they mixed the 4 solutions in varying ratios and spotted their first plate, leaving a spot for the reference solution 1:2:2 Ni:Fe:Co +10% ethylene glycol. The plate will be annealed and then tested in the HARPOON kit next time.
If your team didn’t get an update this week it’s because you didn’t post anything to Google Drive! Please try to remember to post an update to your drive document every single meeting- it’ll help us all stay connected and hopefully improve each other’s work.
Weeks of 10/17-10/30
Many of our teams missed a meeting in the past 2 weeks, so I’ve combined the posts for the weeks.
Oakwood is starting off the school year with new and excited members of the Solar Army! Previously, Oakwood manufactured a UV-ozone cleaner to ultra-clean the FTO glass, making it very hydrophilic. Hydrophilic surfaces allow water to spread evenly instead of forming a droplet. Using a piece of plastic with holes where the spots would eventually be, the UV-ozone treatment allows for perfect placement of our drops while pipetting. This procedure allowed aqueous solutions to dry uniformly on the surface, so the spots don’t have a coffee ring effect. Several other teams are planning on building their own ozone cleaners this year based on Oakwood’s design.
This fall Oakwood is going to make a new template meant for the smaller size glass that SEAL uses. The previous template was for slightly larger glass, and the plastic didn’t hold up well to continued UV-ozone treatment. They are going to try some more stable plastic pieces and see if those are better. They are also going to test how salts of the same metal, but with different counter-ions and non-metal additives, might produce different film properties. They will start by looking at simple iron salts which are readily available.
Concordia group reviewed the conceptual basics of water-splitting catalysts. Their mentor, Dr. Kenny, also described how H2O splitting is similar to photosynthesis/glycolysis in nature. They discussed how metal oxides act as catalysts when splitting H2O, and how acids and bases affect water half-splitting reactions, which result in hydroxides that will be split into water, oxygen, and electrons. If you have any questions about these concepts, ask the Concordia team in the comments section!
Poly is still continuing ahead with work on bismuth nitrate, and PCC is making solutions of bismuth(III) nitrate, lead(II) nitrate, aluminum(III) nitrate, and nickel(II) nitrate based on results of some plates they tested last week. Mayfield’s SEA team is testing combinations of Co and Cu with Al and Zn. RAM team is testing BiCoW combinations since their BiVW plate did not perform well (though their vanadium solution had precipitated out and turned yellow, so it may be a problem with their vanadium).
The San Marino Red Team continued experimentation with Juice from Juice, testing figs, blackberries, blueberries, and green beans as dyes. After assembling the cells, the blue berry dye worked the best (that’s a surprise to me- blackberries usually are better)! They also plan to test spinach leaf extract an raspberries in the future as well as testing if increased surface area of the DSSC affects their results. Red Team also made a plate with various combinations of copper sulfate, nickel nitrate and manganese chloride. San Marino’s Magic Wand Team examined their plate from last time and noticed that a few spots had turned a dark color which they decided was indicative of a solid result. Those spots consisted of the ratios 3:3:4 and 7:1:2 NaH2PO2: KH2PO4: NiCl2. They made another plate that consisted of just these two ratios. Plans to compare dropcasting to electrodeposition for the two materials is put on hold as the “magic wand” for electrodeposition is broken. All the samples were dropcasted this time. The team also talked about semiconductors and band gaps and our hoping these materials they spotted will be metal oxides with a band gap in the “sweet spot” for water oxidation.
Week of 10/10-10/16/16
A few groups are still working on getting started, but if you’ve begun research, be sure to update your team’s Google Drive Doc! This “Week in Review” we have 3 updates from San Marino, Beckman and Franklin.
San Marino has divided into two teams. One team is starting out by working on the Juice from Juice project and seeing what insights they can learn from studying TiO2 semiconductor with various dyes as light absorbers. Team “Magic Wand” is continuing last year’s work on electroplating. They made 1M solutions of NaH2PO2 [Sodium Hypophosphite], KH2PO4 [Potassium Phosphate Monobasic], and NiCl2 [Nickel (II) Chloride] that could be mixed in various combinations to make 8 new samples. These samples were electroplated onto FTO and will be tested at the next meeting.
The Franklin group is also divided into subteams, with each subteam picking 4 metal solutions they would like to work with. This week they reviewed calculations for generating the solutions and got to work mixing up solutions and storing them for future use in dropcasting.
Lastly, the Beckman HS team is tackling the common issue of flaky (and glittery) iron oxide spots. Instead of the 1M solutions they used last team, team members made 0.04M and 0.05M solutions in hopes that these will yield a better product. Other team members started work on using UV light exposure to help with making uniform spots, a technique developed by Oakwood last year and used by several others during the summer program. We’re hoping to write up the plans for the device so that any teams who wish can build their own ozone cleaning device. Comment below if you would be interested in such a device for your team!
Week of 10/3-10/9/16
Welcome to the first “SEAL Week in Review” of the school year! The Google Drive files weren’t available until part-way through the week, so my apologies to those teams who didn’t get a chance to update with their week’s work. A few teams were also struggling with file permissions so I’m sure next week we will have more updates from everyone.
This week’s highlights include our two teams from Irvine- Beckman and Concordia- and the three subgroups from Mayfield:
Beckman HS mostly has new students on their team so they have gone back to the basics, practicing pipetting techniques with iron nitrate. They prepared 4 plates, and ran 2 of them with the SEAL kit. If you have any tips for pipetting or advice for new SEAL members leave them in the comments section!
The Concordia team also has several new members so they have been reviewing the lab techniques for preparing, spotting, and annealing plates. They decided on a naming system for their sample plates (important thing for all teams to consider early on!) and practiced etching the FTO glass so that the sample name is legible. They also considered the best way to clean the glass before depositing the metal solutions. Rinse with water to remove any dust or debris, and then wipe down with acetone or isopropanol- Concordia experimentally determined both work fine- to wipe away any organic impurities on the glass. The first sample they are testing is iron (III) nitrate with bismuth nitrate. Looking forward to hearing about the results!
Lastly, Mayfield is split into three subgroups: RAM, SEA and KEN. The RAM team is continuing work from previous years with bismuth, vanadium and a third metal. They chose tungsten and are testing ratios that have worked well in the past for Bi, V, X combinations- 8:1:1, 8:1:5, 8:1:8. The vanadium solution wasn’t fully dissolved and the spots formed coffee rings after annealing, but the spots did turn a nice bright yellow. Team SEA is working on a testing system to analyze the effect of colored metal salts with non-colored salts. Colored metal oxides should be better at light absorption so they have a pattern to test various combinations that should all be colored in some way. Lastly team KEN is continuing work on their varied ratios template. They have made an iron standard plate to practice and a CoZn sample with the extreme varied ratios pattern.
If you have any questions on the work these groups are doing, leave your question in the comments and hopefully we can start a discussion. Be sure to update your Google Drive file this week so we can get highlights from everyone for next time’s “SEAL Week in Review”!
SEAL Kickoff
Welcome to the SoCal Infantry Blog! Every week I will curate the results and updates from each school and collect it all in a weekly blog post. This will help us all stay connected and able to share ideas before the end of the year SEAL Con. You can post comments, ask and answer questions on the weekly posts.
For this to work, please remember to update your team’s Google Drive doc after each meeting!