Very few updates this past week, but here are highlights from San Marino HS and catch-up updates from Team 3 (“Las Chemists”) from Franklin HS.
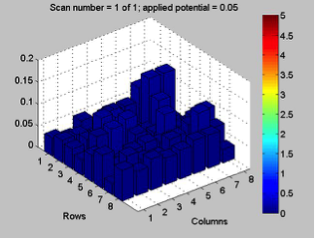
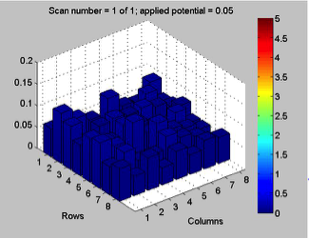
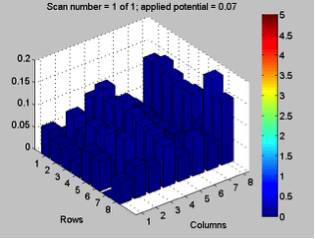
Red team from San Marino tested their plate from last time containing various combinations of 0.1 Molar of MnCl2, CuSO4, and Ni(NO3)2. None of the metal oxide spots resulted in higher current than the blank spots. They also planned to test blackberry juice as a dye in DSSCs with large and small surface areas of TiO2. Unfortunately they weren’t able to complete both tests before the sun set. Magic Wand Team tested their plate from last week with the dropcast Ni2P3 and NiP4 solutions. After putting the FTO plate in the kiln, the spots changed color from black to a green-brown (as shown below). They did three scans, the first two with an applied potential of 0.05, and the third with an applied potential of 0.07 (as shown below). None of the scans showed high current in any of the materials. Then, they made another FTO plate with the same solutions to test how different amounts of time on the hot plate could affect the solutions. Unfortunately the FTO plate shattered due to repeated changing temperatures of the hot plate and the lab table.
One of Franklin’s subgroups, Las Chemists, has documented their work so far this year on Google Drive. They decided on 4 metal salts to make solutions of: sodium molybdate dihydrate, cobalt chloride hexahydrate, nickel nitrate hexahydrate, and cobalt acetate tetrahydrate. After making their solutions, they also added 10% ethylene glycol to each solution (assuming for easier spotting? Let us know in the comments ladies!). The they mixed the 4 solutions in varying ratios and spotted their first plate, leaving a spot for the reference solution 1:2:2 Ni:Fe:Co +10% ethylene glycol. The plate will be annealed and then tested in the HARPOON kit next time.
If your team didn’t get an update this week it’s because you didn’t post anything to Google Drive! Please try to remember to post an update to your drive document every single meeting- it’ll help us all stay connected and hopefully improve each other’s work.

My question is for the Las Chemists from Franklin HS: Why did you guys use the 10% ethylene glycol?
We were going to originally use glycerol but we used the ethylene to change the viscosity of our solutions so it would evaporate evenly.
In previous tests, did the differences in applied potential have a significant effect upon results?
This is the first test we ran with these chemicals. But we did take the chemicals and portions into consideration.
Differences in applied potential did not have a significant effect on results in terms of spikes on certain spots. However, what the effect of applied potential on dark current did tell us is whether or not something was corroding. We can quickly discount results of a test if the dark current is rapidly “running away” from us as we try to adjust the applied potential.
Since their plate was not successful, what are they planning to change in order to improve results?
We accidentally did it on the wrong side of the plate. So we will be starting all over from drop casting our references on the right side and our references solution has 10% more glycerol.